Detection of drug resistant Mycobacterium tuberculosis by high-throughput sequencing of DNA isolated from acid fast bacilli smears
- PMID: 32384098
- PMCID: PMC7209238
- DOI: 10.1371/journal.pone.0232343
Detection of drug resistant Mycobacterium tuberculosis by high-throughput sequencing of DNA isolated from acid fast bacilli smears
Abstract
Background: Drug susceptibility testing for Mycobacterium tuberculosis (MTB) is difficult to perform in resource-limited settings where Acid Fast Bacilli (AFB) smears are commonly used for disease diagnosis and monitoring. We developed a simple method for extraction of MTB DNA from AFB smears for sequencing-based detection of mutations associated with resistance to all first and several second-line anti-tuberculosis drugs.
Methods: We isolated MTB DNA by boiling smear content in a Chelex solution, followed by column purification. We sequenced PCR-amplified segments of the rpoB, katG, embB, gyrA, gyrB, rpsL, and rrs genes, the inhA, eis, and pncA promoters and the entire pncA gene.
Results: We tested our assay on 1,208 clinically obtained AFB smears from Ghana (n = 379), Kenya (n = 517), Uganda (n = 262), and Zambia (n = 50). Coverage depth varied by target and slide smear grade, ranging from 300X to 12000X on average. Coverage of ≥20X was obtained for all targets in 870 (72%) slides overall. Mono-resistance (5.9%), multi-drug resistance (1.8%), and poly-resistance (2.4%) mutation profiles were detected in 10% of slides overall, and in over 32% of retreatment and follow-up cases.
Conclusion: This rapid AFB smear DNA-based method for determining drug resistance may be useful for the diagnosis and surveillance of drug-resistant tuberculosis.
Conflict of interest statement
M.R. owns shares of Illumina, Inc.; D.A. and his laboratory receive licensing fees for use of primers and probes in molecular diagnostic assays from Cepheid Inc. Cepheid also provides research support in the form of grants, equipment, and consumables to D.A.’s laboratory. D.A. is listed as an inventor for US patent applications PCT/US18/48611 Therapeutic Indazoles and PCT/US18/48607 Therapeutic Indoles for treating tuberculosis; S.C. is employed by Cepheid, which makes diagnostic tests for identifying drug resistant Mycobacterium tuberculosis; N.A. is a member of the Advisory Committee for the Elimination of Tuberculosis (ACET) for the Centers for Disease Control and Prevention (CDC); all other authors report no competing interests. These affiliations do not alter our adherence toPLOS ONE policies on sharing data and materials.
Figures
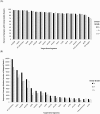
Similar articles
-
Application of next-generation sequencing to detect variants of drug-resistant Mycobacterium tuberculosis: genotype-phenotype correlation.Ann Clin Microbiol Antimicrob. 2019 Jan 3;18(1):2. doi: 10.1186/s12941-018-0300-y. Ann Clin Microbiol Antimicrob. 2019. PMID: 30606210 Free PMC article.
-
Comparison of targeted next-generation sequencing and the Xpert MTB/RIF assay for detection of Mycobacterium tuberculosis in clinical isolates and sputum specimens.Microbiol Spectr. 2024 May 2;12(5):e0409823. doi: 10.1128/spectrum.04098-23. Epub 2024 Apr 11. Microbiol Spectr. 2024. PMID: 38602399 Free PMC article.
-
Molecular characterization of mutations associated with resistance to second-line tuberculosis drug among multidrug-resistant tuberculosis patients from high prevalence tuberculosis city in Morocco.BMC Infect Dis. 2018 Feb 27;18(1):98. doi: 10.1186/s12879-018-3009-9. BMC Infect Dis. 2018. PMID: 29486710 Free PMC article.
-
[Rapid diagnosis of tuberculosis].Kekkaku. 1997 Dec;72(12):659-72. Kekkaku. 1997. PMID: 9465560 Review. Japanese.
-
[Multidrug-resistant tuberculosis. 2. Mechanisms of drug-resistance in Mycobacterium tuberculosis--genetic mechanisms of drug-resistance].Kekkaku. 1998 Nov;73(11):657-63. Kekkaku. 1998. PMID: 9866928 Review. Japanese.
Cited by
-
Deep Amplicon Sequencing Reveals Culture-dependent Clonal Selection of Mycobacterium tuberculosis in Clinical Samples.Genomics Proteomics Bioinformatics. 2025 Jan 15;22(6):qzae046. doi: 10.1093/gpbjnl/qzae046. Genomics Proteomics Bioinformatics. 2025. PMID: 38870522 Free PMC article.
-
Enhanced diagnosis of multi-drug-resistant microbes using group association modeling and machine learning.Nat Commun. 2025 Mar 25;16(1):2933. doi: 10.1038/s41467-025-58214-6. Nat Commun. 2025. PMID: 40133304 Free PMC article.
-
Exploring gene mutations and multidrug resistance in Mycobacterium tuberculosis: a study from the Lung Hospital in Vietnam.Mol Biol Rep. 2024 Oct 21;51(1):1084. doi: 10.1007/s11033-024-10015-8. Mol Biol Rep. 2024. PMID: 39432118
-
Targeted next-generation sequencing to diagnose drug-resistant tuberculosis: a systematic review and meta-analysis.Lancet Infect Dis. 2024 Oct;24(10):1162-1176. doi: 10.1016/S1473-3099(24)00263-9. Epub 2024 May 22. Lancet Infect Dis. 2024. PMID: 38795712 Free PMC article.
-
Revisiting the methods for detecting Mycobacterium tuberculosis: what has the new millennium brought thus far?Access Microbiol. 2021 Aug 2;3(8):000245. doi: 10.1099/acmi.0.000245. eCollection 2021. Access Microbiol. 2021. PMID: 34595396 Free PMC article. Review.
References
-
- World Health Organization. Global tuberculosis report 2018. Geneva: 2018. Licence: CC BY-NC-SA 3.0 IGO. http://apps.who.int/iris/bitstream/handle/10665/274453/9789241565646-eng.... Accessed December 1, 2018.
-
- World Health Organization. Rapid communication: key changes to treatment of multidrug- and rifampicin-resistant tuberculosis (MDR/RR-TB). Geneva: 2018. Licence: CC BY-NC-SA 3.0 IGO. https://www.who.int/tb/publications/2018/WHO_RapidCommunicationMDRTB.pdf.... Accessed December 1, 2018.
-
- World Health Organization. Global tuberculosis report 2017. Geneva: 2017. Licence: CC BY-NCSA 3.0 IGO. http://apps.who.int/medicinedocs/documents/s23360en/s23360en.pdf. Accessed December 1, 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

