Impact of arginine therapy on mitochondrial function in children with sickle cell disease during vaso-occlusive pain
- PMID: 32384147
- PMCID: PMC7498366
- DOI: 10.1182/blood.2019003672
Impact of arginine therapy on mitochondrial function in children with sickle cell disease during vaso-occlusive pain
Abstract
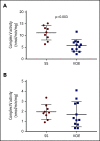
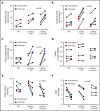
Altered mitochondrial function occurs in sickle cell disease (SCD), due in part to low nitric oxide (NO) bioavailability. Arginine, the substrate for NO production, becomes acutely deficient in SCD patients with vaso-occlusive pain episodes (VOE). To determine if arginine improves mitochondrial function, 12 children with SCD-VOE (13.6 ± 3 years; 67% male; 75% hemoglobin-SS) were randomized to 1 of 3 arginine doses: (1) 100 mg/kg IV 3 times/day (TID); (2) loading dose (200 mg/kg) then 100 mg/kg TID; or (3) loading dose (200 mg/kg) followed by continuous infusion (300 mg/kg per day) until discharge. Platelet-rich plasma mitochondrial activity, protein expression, and protein-carbonyls were measured from emergency department (ED) presentation vs discharge. All VOE subjects at ED presentation had significantly decreased complex-V activity compared to a steady-state cohort. Notably, complex-V activity was increased at discharge in subjects from all 3 arginine-dosing schemes; greatest increase occurred with a loading dose (P < .001). Although complex-IV and citrate synthase activities were similar in VOE platelets vs steady state, enzyme activities were significantly increased in VOE subjects after arginine-loading dose treatment. Arginine also decreased protein-carbonyl levels across all treatment doses (P < .01), suggesting a decrease in oxidative stress. Arginine therapy increases mitochondrial activity and reduces oxidative stress in children with SCD/VOE. This trial was registered at www.clinicaltrials.gov as #NCT02536170.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: C.R.M. is the inventor or coinventor of several University of California San Francisco-Benioff Children’s Hospital Oakland patents/patent-pending applications that include nutritional supplements and biomarkers of cardiovascular disease related to arginine bioavailability, is an inventor of an Emory University School of Medicine patent application for a nutritional supplement, is a consultant for Pfizer, and has received research support from MAST Therapeutics, the US Food and Drug Administration, and the National Institutes of Health. C.D.D. has received research support from Pfizer. The remaining authors declare no competing financial interests.
Figures
Comment in
-
Arginine for mitochondrial oxidative enzymopathy.Blood. 2020 Sep 17;136(12):1376-1378. doi: 10.1182/blood.2020006732. Blood. 2020. PMID: 32941632 Free PMC article.
References
-
- Brousseau DC, Owens PL, Mosso AL, Panepinto JA, Steiner CA. Acute care utilization and rehospitalizations for sickle cell disease. JAMA. 2010;303(13):1288-1294. - PubMed
-
- Carden MA, Brousseau DC, Ahmad FA, et al. ; Sickle Cell Disease Arginine Study Group and the Pediatric Emergency Care Applied Research Network (PECARN) . Normal saline bolus use in pediatric emergency departments is associated with poorer pain control in children with sickle cell anemia and vaso-occlusive pain. Am J Hematol. 2019;94(6):689-696. - PMC - PubMed
-
- Carden MA, Patil P, Ahmad ME, Lam WA, Joiner CH, Morris CR. Variations in pediatric emergency medicine physician practices for intravenous fluid management in children with sickle cell disease and vaso-occlusive pain: a single institution experience. Pediatr Blood Cancer. 2018;65(1):65. - PubMed
-
- Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312(10):1033-1048. - PubMed

