Survival following allogeneic transplant in patients with myelofibrosis
- PMID: 32384540
- PMCID: PMC7218417
- DOI: 10.1182/bloodadvances.2019001084
Survival following allogeneic transplant in patients with myelofibrosis
Erratum in
-
Gowin K, Ballen K, Ahn KW, et al. Survival following allogeneic transplant in patients with myelofibrosis. Blood Adv. 2020;4(9):1965-1973.Blood Adv. 2021 Jul 13;5(13):2751. doi: 10.1182/bloodadvances.2021005191. Blood Adv. 2021. PMID: 34242390 Free PMC article. No abstract available.
Abstract
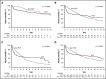
Allogeneic hematopoietic cell transplantation (HCT) is the only curative therapy for myelofibrosis (MF). In this large multicenter retrospective study, overall survival (OS) in MF patients treated with allogeneic HCT (551 patients) and without HCT (non-HCT) (1377 patients) was analyzed with Cox proportional hazards model. Survival analysis stratified by the Dynamic International Prognostic Scoring System (DIPSS) revealed that the first year of treatment arm assignment, due to upfront risk of transplant-related mortality (TRM), HCT was associated with inferior OS compared with non-HCT (non-HCT vs HCT: DIPSS intermediate 1 [Int-1]: hazard ratio [HR] = 0.26, P < .0001; DIPSS-Int-2 and higher: HR, 0.39, P < .0001). Similarly, in the DIPSS low-risk MF group, due to upfront TRM risk, OS was superior with non-HCT therapies compared with HCT in the first-year post treatment arm assignment (HR, 0.16, P = .006). However, after 1 year, OS was not significantly different (HR, 1.38, P = .451). Beyond 1 year of treatment arm assignment, an OS advantage with HCT therapy in Int-1 and higher DIPSS score patients was observed (non-HCT vs HCT: DIPSS-Int-1: HR, 2.64, P < .0001; DIPSS-Int-2 and higher: HR, 2.55, P < .0001). In conclusion, long-term OS advantage with HCT was observed for patients with Int-1 or higher risk MF, but at the cost of early TRM. The magnitude of OS benefit with HCT increased as DIPSS risk score increased and became apparent with longer follow-up.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: A.T.K. received research funding from Incyte, AbbVie, Janssen, and Colgene. A.T.G. received research funding from Celgene, Incyte, CTI Biopharma, and Pfizer. G.H. received research funding from Bayer, Meirck, Incyte, and Celgene. R.F.O. received research funding from AstraZeneca. Z.D. received research funding from Incyte Corp. M.O.A. received research funding from Incyte, Gilead, Samus Therapeautics, CTI Biopharma, and Janssen. B.R.A. contributed to Best Practice guidelines for the
Figures
References
-
- Arber DA, Orazi A, Hasserjian R, et al. . The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405. - PubMed
-
- Levine RL, Wadleigh M, Cools J, et al. . Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387-397. - PubMed
-
- Kralovics R, Passamonti F, Buser AS, et al. . A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779-1790. - PubMed
-
- James C, Ugo V, Le Couédic JP, et al. . A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144-1148. - PubMed
Publication types
MeSH terms
Grants and funding
- U01 AI126612/AI/NIAID NIH HHS/United States
- R01 CA231141/CA/NCI NIH HHS/United States
- U01 HL128568/HL/NHLBI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- R01 HL129472/HL/NHLBI NIH HHS/United States
- P01 CA111412/CA/NCI NIH HHS/United States
- P30 CA054174/CA/NCI NIH HHS/United States
- R01 HL131731/HL/NHLBI NIH HHS/United States
- R01 HL126589/HL/NHLBI NIH HHS/United States
- R01 CA152108/CA/NCI NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- R21 HL140314/HL/NHLBI NIH HHS/United States
- U24 CA076518/CA/NCI NIH HHS/United States
- U24 HL138660/HL/NHLBI NIH HHS/United States

