Mesenchymal stromal cell therapeutic potency is dependent upon viability, route of delivery, and immune match
- PMID: 32384543
- PMCID: PMC7218435
- DOI: 10.1182/bloodadvances.2020001711
Mesenchymal stromal cell therapeutic potency is dependent upon viability, route of delivery, and immune match
Abstract
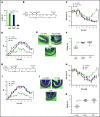
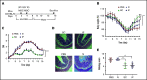
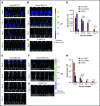
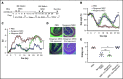
Culture-adapted bone marrow mesenchymal stromal cells (MSCs) deploy paracrine anti-inflammatory and tissue regenerative functionalities that can be harnessed as a living cell pharmaceutical product. Independent of clinical indication, a near majority of human clinical trials administer MSC IV, often with an allogeneic MSC cell product immediately after thawing from cryostorage. Despite hundreds of studies in a wide assortment of inflammatory, degenerative, and acute tissue injury syndromes, human clinical outcomes often fail to mirror promising rigorously conducted preclinical animal studies. Using a mouse model of toxic colitis, we demonstrate that replication fit MSCs harvested in log phase of growth have substantial impact on colitis clinical and pathologic endpoints when delivered subcutaneously or intraperitoneally, whereas the maximum tolerated IV bolus dosing failed to do so. We also demonstrate that heat-inactivated MSCs lose all therapeutic utility and the observation is mirrored by use of viable MSC administered immediately postthaw from cryostorage. Using luciferase transgenic MSC as donor cells, we demonstrate that transient in vivo engraftment is severely compromised when MSCs are dead or thawed and further demonstrate that MSC redosing is feasible in relapsing colitis, but only syngeneic MSCs lead to sustained improvement of clinical endpoints. These data support the notion that pharmaceutical potency of MSC requires viability and functional fitness. Reciprocally, IV administration of thawed MSC products may be biased against positive clinical outcomes for treatment of colitis and that extravascular administration of syngeneic, fit MSCs allows for effect in a recurrent therapy model.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

Similar articles
-
Evaluation of Porcine Versus Human Mesenchymal Stromal Cells From Three Distinct Donor Locations for Cytotherapy.Front Immunol. 2020 May 6;11:826. doi: 10.3389/fimmu.2020.00826. eCollection 2020. Front Immunol. 2020. PMID: 32435248 Free PMC article.
-
Intramuscular administration potentiates extended dwell time of mesenchymal stromal cells compared to other routes.Cytotherapy. 2018 Feb;20(2):232-244. doi: 10.1016/j.jcyt.2017.09.013. Epub 2017 Nov 20. Cytotherapy. 2018. PMID: 29167063
-
Rapid and effective preparation of clonal bone marrow-derived mesenchymal stem/stromal cell sheets to reduce renal fibrosis.Sci Rep. 2023 Mar 17;13(1):4421. doi: 10.1038/s41598-023-31437-7. Sci Rep. 2023. PMID: 36932137 Free PMC article.
-
Chimerism of bone marrow mesenchymal stem/stromal cells in allogeneic hematopoietic cell transplantation: is it clinically relevant?Chimerism. 2013 Jul-Sep;4(3):78-83. doi: 10.4161/chim.25609. Epub 2013 Jul 11. Chimerism. 2013. PMID: 23880502 Free PMC article. Review.
-
Tolerance to Bone Marrow Transplantation: Do Mesenchymal Stromal Cells Still Have a Future for Acute or Chronic GvHD?Front Immunol. 2020 Dec 11;11:609063. doi: 10.3389/fimmu.2020.609063. eCollection 2020. Front Immunol. 2020. PMID: 33362797 Free PMC article. Review.
Cited by
-
The Current Status of Mesenchymal Stromal Cells: Controversies, Unresolved Issues and Some Promising Solutions to Improve Their Therapeutic Efficacy.Front Cell Dev Biol. 2021 Mar 16;9:650664. doi: 10.3389/fcell.2021.650664. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33796536 Free PMC article. Review.
-
Cell Therapies for Acute Radiation Syndrome.Int J Mol Sci. 2024 Jun 26;25(13):6973. doi: 10.3390/ijms25136973. Int J Mol Sci. 2024. PMID: 39000080 Free PMC article. Review.
-
MSC Therapies for COVID-19: Importance of Patient Coagulopathy, Thromboprophylaxis, Cell Product Quality and Mode of Delivery for Treatment Safety and Efficacy.Front Immunol. 2020 May 19;11:1091. doi: 10.3389/fimmu.2020.01091. eCollection 2020. Front Immunol. 2020. PMID: 32574263 Free PMC article.
-
MSCs and Inflammatory Cells Crosstalk in Regenerative Medicine: Concerted Actions for Optimized Resolution Driven by Energy Metabolism.Front Immunol. 2021 Apr 30;12:626755. doi: 10.3389/fimmu.2021.626755. eCollection 2021. Front Immunol. 2021. PMID: 33995350 Free PMC article. Review.
-
Therapeutic Potential and Mechanisms of Mesenchymal Stem Cells in Coronary Artery Disease: Narrative Review.Int J Mol Sci. 2025 Jun 5;26(11):5414. doi: 10.3390/ijms26115414. Int J Mol Sci. 2025. PMID: 40508220 Free PMC article. Review.
References
-
- Shi Y, Wang Y, Li Q, et al. . Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14(8):493-507. - PubMed
-
- Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15(11):1009-1016. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

