Chronic Kidney Disease-Associated Immune Dysfunctions: Impact of Protein-Bound Uremic Retention Solutes on Immune Cells
- PMID: 32384617
- PMCID: PMC7291164
- DOI: 10.3390/toxins12050300
Chronic Kidney Disease-Associated Immune Dysfunctions: Impact of Protein-Bound Uremic Retention Solutes on Immune Cells
Abstract
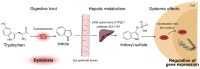
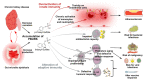
Regardless of the primary disease responsible for kidney failure, patients suffering from chronic kidney disease (CKD) have in common multiple impairments of both the innate and adaptive immune systems, the pathophysiology of which has long remained enigmatic. CKD-associated immune dysfunction includes chronic low-grade activation of monocytes and neutrophils, which induces endothelial damage and increases cardiovascular risk. Although innate immune effectors are activated during CKD, their anti-bacterial capacity is impaired, leading to increased susceptibility to extracellular bacterial infections. Finally, CKD patients are also characterized by profound alterations of cellular and humoral adaptive immune responses, which account for an increased risk for malignancies and viral infections. This review summarizes the recent emerging data that link the pathophysiology of CKD-associated immune dysfunctions with the accumulation of microbiota-derived metabolites, including indoxyl sulfate and p-cresyl sulfate, the two best characterized protein-bound uremic retention solutes.
Keywords: chronic kidney disease; immune system; uremic toxins.
Conflict of interest statement
All the authors declared no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

