Carbapenemases: Transforming Acinetobacter baumannii into a Yet More Dangerous Menace
- PMID: 32384624
- PMCID: PMC7277208
- DOI: 10.3390/biom10050720
Carbapenemases: Transforming Acinetobacter baumannii into a Yet More Dangerous Menace
Abstract
Acinetobacter baumannii is a common cause of serious nosocomial infections. Although community-acquired infections are observed, the vast majority occur in people with preexisting comorbidities. A. baumannii emerged as a problematic pathogen in the 1980s when an increase in virulence, difficulty in treatment due to drug resistance, and opportunities for infection turned it into one of the most important threats to human health. Some of the clinical manifestations of A. baumannii nosocomial infection are pneumonia; bloodstream infections; lower respiratory tract, urinary tract, and wound infections; burn infections; skin and soft tissue infections (including necrotizing fasciitis); meningitis; osteomyelitis; and endocarditis. A. baumannii has an extraordinary genetic plasticity that results in a high capacity to acquire antimicrobial resistance traits. In particular, acquisition of resistance to carbapenems, which are among the antimicrobials of last resort for treatment of multidrug infections, is increasing among A. baumannii strains compounding the problem of nosocomial infections caused by this pathogen. It is not uncommon to find multidrug-resistant (MDR, resistance to at least three classes of antimicrobials), extensively drug-resistant (XDR, MDR plus resistance to carbapenems), and pan-drug-resistant (PDR, XDR plus resistance to polymyxins) nosocomial isolates that are hard to treat with the currently available drugs. In this article we review the acquired resistance to carbapenems by A. baumannii. We describe the enzymes within the OXA, NDM, VIM, IMP, and KPC groups of carbapenemases and the coding genes found in A. baumannii clinical isolates.
Keywords: Acinetobacter; ESKAPE; antibiotic resistance; plasmid; β-lactam; β-lactamase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

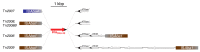

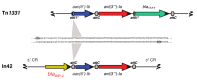
References
-
- Hartstein A.I., Rashad A.L., Liebler J.M., Actis L.A., Freeman J., Rourke J.W., Jr., Stibolt T.B., Tolmasky M.E., Ellis G.R., Crosa J.H. Multiple intensive care unit outbreak of Acinetobacter calcoaceticus subspecies anitratus respiratory infection and colonization associated with contaminated, reusable ventilator circuits and resuscitation bags. Am. J. Med. 1988;85:624–631. doi: 10.1016/S0002-9343(88)80233-X. - DOI - PubMed
-
- Hartstein A.I., Morthland V.H., Rourke J.W., Jr., Freeman J., Garber S., Sykes R., Rashad A.L. Plasmid DNA fingerprinting of Acinetobacter calcoaceticus subspecies anitratus from intubated and mechanically ventilated patients. Infect. Control. Hosp. Epidemiol. 1990;11:531–538. doi: 10.2307/30151321. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

