The Advantages of Clinical Nutrition Use in Oncologic Patients in Italy: Real World Insights
- PMID: 32384639
- PMCID: PMC7349883
- DOI: 10.3390/healthcare8020125
The Advantages of Clinical Nutrition Use in Oncologic Patients in Italy: Real World Insights
Abstract
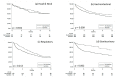
This retrospective observational study aimed to provide insights on the use of clinical nutrition (CN) (enteral and parenteral feeding) and outcomes in an Italian real-world setting. The data source comes from administrative databases of 10 Italian Local Health Units. Patients diagnosed with malignant neoplasms from 1 January 2010 to 31 December 2015 were included. Metastasis presence was ascertained by discharge diagnosis in the hospitalization database. CN was identified by specific codes from pharmaceutical and hospitalization databases. Two cohorts were created-one for metastatic patients (N = 53,042), and one for non-metastatic patients (N = 4379) receiving CN. Two survival analyses were set for the cohort of metastatic patients-one included patients receiving CN and the second included malnourished patients. Our findings show that (1) administration of CN is associated with positive survival outcomes in metastatic patients with gastrointestinal, respiratory, and genitourinary cancer; (2) CN in malnourished metastatic patients with gastrointestinal and genitourinary cancer was associated with significant improvement in survival; (3) early administration of CN was associated with improvement in survival in non-metastatic patients with gastrointestinal cancer (HR 95%CI: 0.5 (0.4-0.6), p-value < 0.05). This study highlights the need to improve the assessment of nutritional status in oncologic patients and suggests a potential survival benefit of CN treatment in metastatic disease.
Keywords: clinical nutrition; malnutrition; metastasis; oncology; real-world.
Conflict of interest statement
P.P. reports speakers’ honoraria from Baxter Healthcare Corporation. R.C. reports speakers’ honoraria and research grants from Baxter Healthcare Corporation. P.C. reports speakers’ honoraria from Baxter Healthcare Corporation. F.D.C. reports consultancy for Baxter Healthcare Corporation. A.S. reports: advisory board work for BMS (Bristol-Myers Squibb), Servier, Gilead, Pfizer, Eisai, Bayer, MSD (Merck Sharp & Dohme); consultancy for Arqule; Speaker’s Bureau for Takeda BMS Roche Abb-Vie, Amgen, Celgene, Servier, Gilead, Astrazeneca, Pfizer, Arqule, Lilly, Sandoz, Eisai, Novartis, Bayer, MSD. C.G., C.P. have declared no conflicts of interest. L.D.E, V.P., D.S. report no conflict of interest. Clicon S.r.l. is an independent company. The agreement signed by Clicon S.r.l. and Baxter Healthcare Corporation does not create any entityship, joint venture, or any similar relationship between parties. Neither CliCon S.r.l. nor any of their representatives are employees of Baxter Healthcare Corporation for any purpose. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Trestini I., Carbognin L., Sperduti I., Bonaiuto C., Auriemma A., Melisi D., Salvatore L., Bria E., Tortora G. Prognostic impact of early nutritional support in patients affected by locally advanced and metastatic pancreatic ductal adenocarcinoma undergoing chemotherapy. Eur. J. Clin. Nutr. 2018;72:772–779. doi: 10.1038/s41430-018-0155-5. - DOI - PubMed
-
- Caccialanza R., Pedrazzoli P., Cereda E., Gavazzi C., Pinto C., Paccagnella A., Beretta G.D., Nardi M., Laviano A., Zagonel V. Nutritional Support in Cancer Patients: A Position Paper from the Italian Society of Medical Oncology (AIOM) and the Italian Society of Artificial Nutrition and Metabolism (SINPE) J. Cancer. 2016;7:131–135. doi: 10.7150/jca.13818. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

