Transcutaneous Administration of Dengue Vaccines
- PMID: 32384822
- PMCID: PMC7290698
- DOI: 10.3390/v12050514
Transcutaneous Administration of Dengue Vaccines
Abstract
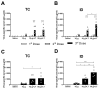
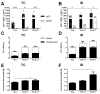
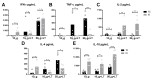
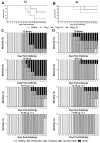
In the present study, we evaluated the immunological responses induced by dengue vaccines under experimental conditions after delivery via a transcutaneous (TC) route. Vaccines against type 2 Dengue virus particles (DENV2 New Guinea C (NGC) strain) combined with enterotoxigenic Escherichia coli (ETEC) heat-labile toxin (LT) were administered to BALB/c mice in a three-dose immunization regimen via the TC route. As a control for the parenteral administration route, other mouse groups were immunized with the same vaccine formulation via the intradermic (ID) route. Our results showed that mice vaccinated either via the TC or ID routes developed similar protective immunity, as measured after lethal challenges with the DENV2 NGC strain. Notably, the vaccine delivered through the TC route induced lower serum antibody (IgG) responses with regard to ID-immunized mice, particularly after the third dose. The protective immunity elicited in TC-immunized mice was attributed to different antigen-specific antibody properties, such as epitope specificity and IgG subclass responses, and cellular immune responses, as determined by cytokine secretion profiles. Altogether, the results of the present study demonstrate the immunogenicity and protective properties of a dengue vaccine delivered through the TC route and offer perspectives for future clinical applications.
Keywords: adjuvant; dengue vaccines; heat-labile toxin; intradermic immunization; transcutaneous immunization.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- World Health Organization (WHO) Dengue and severe dengue. [(accessed on 8 May 2019)]; Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue.
-
- Glenn G.M., Scharton-Kersten T., Vassell R., Mallett C.P., Hale T.L., Alving C.R. Transcutaneous immunization with cholera toxin protects mice against lethal mucosal toxin challenge. J. Immunol. 1998;161:3211–3214. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

