SMN-deficiency disrupts SERCA2 expression and intracellular Ca2+ signaling in cardiomyocytes from SMA mice and patient-derived iPSCs
- PMID: 32384912
- PMCID: PMC7206821
- DOI: 10.1186/s13395-020-00232-7
SMN-deficiency disrupts SERCA2 expression and intracellular Ca2+ signaling in cardiomyocytes from SMA mice and patient-derived iPSCs
Abstract
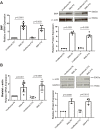
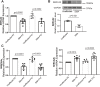
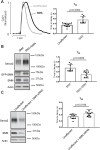
Spinal muscular atrophy (SMA) is a neurodegenerative disease characterized by loss of alpha motor neurons and skeletal muscle atrophy. The disease is caused by mutations of the SMN1 gene that result in reduced functional expression of survival motor neuron (SMN) protein. SMN is ubiquitously expressed, and there have been reports of cardiovascular dysfunction in the most severe SMA patients and animal models of the disease. In this study, we directly assessed the function of cardiomyocytes isolated from a severe SMA model mouse and cardiomyocytes generated from patient-derived IPSCs. Consistent with impaired cardiovascular function at the very early disease stages in mice, heart failure markers such as brain natriuretic peptide were significantly elevated. Functionally, cardiomyocyte relaxation kinetics were markedly slowed and the T50 for Ca2+ sequestration increased to 146 ± 4 ms in SMN-deficient cardiomyocytes from 126 ± 4 ms in wild type cells. Reducing SMN levels in cardiomyocytes from control patient IPSCs slowed calcium reuptake similar to SMA patent-derived cardiac cells. Importantly, restoring SMN increased calcium reuptake rate. Taken together, these results indicate that SMN deficiency impairs cardiomyocyte function at least partially through intracellular Ca2+ cycling dysregulation.
Conflict of interest statement
The authors declare they have no competing interests.
Figures



Similar articles
-
Transcriptional reprogramming in SMA mouse hearts reveals signatures of early heart failure and dysregulated calcium signaling.Hum Mol Genet. 2025 Jun 18;34(13):1123-1133. doi: 10.1093/hmg/ddaf060. Hum Mol Genet. 2025. PMID: 40287831
-
SMN deficiency alters Nrxn2 expression and splicing in zebrafish and mouse models of spinal muscular atrophy.Hum Mol Genet. 2014 Apr 1;23(7):1754-70. doi: 10.1093/hmg/ddt567. Epub 2013 Nov 11. Hum Mol Genet. 2014. PMID: 24218366
-
Decreased Motor Neuron Support by SMA Astrocytes due to Diminished MCP1 Secretion.J Neurosci. 2017 May 24;37(21):5309-5318. doi: 10.1523/JNEUROSCI.3472-16.2017. Epub 2017 Apr 27. J Neurosci. 2017. PMID: 28450545 Free PMC article.
-
The Biochemistry of Survival Motor Neuron Protein Is Paving the Way to Novel Therapies for Spinal Muscle Atrophy.Biochemistry. 2020 Apr 14;59(14):1391-1397. doi: 10.1021/acs.biochem.9b01124. Epub 2020 Apr 2. Biochemistry. 2020. PMID: 32227847 Review.
-
Spinal muscular atrophy and the antiapoptotic role of survival of motor neuron (SMN) protein.Mol Neurobiol. 2013 Apr;47(2):821-32. doi: 10.1007/s12035-013-8399-5. Epub 2013 Jan 13. Mol Neurobiol. 2013. PMID: 23315303 Review.
Cited by
-
Hepatocyte-intrinsic SMN deficiency drives metabolic dysfunction and liver steatosis in spinal muscular atrophy.J Clin Invest. 2024 May 9;134(12):e173702. doi: 10.1172/JCI173702. J Clin Invest. 2024. PMID: 38722695 Free PMC article.
-
Revealing Calcium Signaling Pathway as Novel Mechanism of Danhong Injection for Treating Acute Myocardial Infarction by Systems Pharmacology and Experiment Validation.Front Pharmacol. 2022 Feb 23;13:839936. doi: 10.3389/fphar.2022.839936. eCollection 2022. Front Pharmacol. 2022. PMID: 35281886 Free PMC article.
-
Magnetic resonance reveals mitochondrial dysfunction and muscle remodelling in spinal muscular atrophy.Brain. 2022 May 24;145(4):1422-1435. doi: 10.1093/brain/awab411. Brain. 2022. PMID: 34788410 Free PMC article.
-
Inhibition of minor intron splicing reduces Na+ and Ca2+ channel expression and function in cardiomyocytes.J Cell Sci. 2022 Jan 1;135(1):jcs259191. doi: 10.1242/jcs.259191. Epub 2022 Jan 7. J Cell Sci. 2022. PMID: 34859816 Free PMC article.
-
Transcriptional reprogramming in SMA mouse hearts reveals signatures of early heart failure and dysregulated calcium signaling.Hum Mol Genet. 2025 Jun 18;34(13):1123-1133. doi: 10.1093/hmg/ddaf060. Hum Mol Genet. 2025. PMID: 40287831
References
-
- Feldkotter M, Schwarzer V, Wirth R, Wienker TF, Wirth B. Quantitative analyses of SMN1 and SMN2 based on real-time lightCycler PCR: fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am J Hum Genet. 2002;70(2):358–368. doi: 10.1086/338627. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

