Lactation Versus Formula Feeding: Insulin, Glucose, and Fatty Acid Metabolism During the Postpartum Period
- PMID: 32385056
- PMCID: PMC7372076
- DOI: 10.2337/db19-1226
Lactation Versus Formula Feeding: Insulin, Glucose, and Fatty Acid Metabolism During the Postpartum Period
Abstract
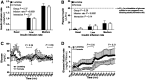
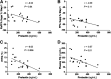
Milk production may involve a transient development of insulin resistance in nonmammary tissues to support redistribution of maternal macronutrients to match the requirements of the lactating mammary gland. In the current study, adipose and liver metabolic responses were measured in the fasting state and during a two-step (10 and 20 mU/m2/min) hyperinsulinemic-euglycemic clamp with stable isotopes, in 6-week postpartum women who were lactating (n = 12) or formula-feeding (n = 6) their infants and who were closely matched for baseline characteristics (e.g., parity, body composition, and intrahepatic lipid). When controlling for the low insulin concentrations of both groups, the lactating women exhibited a fasting rate of endogenous glucose production (EGP) that was 2.6-fold greater and a lipolysis rate that was 2.3-fold greater than the formula-feeding group. During the clamp, the groups exhibited similar suppression rates of EGP and lipolysis. In the lactating women only, higher prolactin concentrations were associated with greater suppression rates of lipolysis and lower intrahepatic lipid and plasma triacylglycerol concentrations. These data suggest that whole-body alterations in glucose transport may be organ specific and facilitate nutrient partitioning during lactation. Recapitulating a shift toward noninsulin-mediated glucose uptake could be an early postpartum strategy to enhance lactation success in women at risk for delayed onset of milk production.
© 2020 by the American Diabetes Association.
Figures




References
-
- Catalano PM, Tyzbir ED, Wolfe RR, et al. . Carbohydrate metabolism during pregnancy in control subjects and women with gestational diabetes. Am J Physiol 1993;264:E60–E67 - PubMed
-
- Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol 1999;180:903–916 - PubMed
-
- Neville MC, Casey C, Hay WW Jr. Endocrine regulation of nutrient flux in the lactating woman. Do the mechanisms differ from pregnancy? In Nutrient Regulation During Pregnancy, Lactation, and Infant Growth. Allen L, King J, Lonnerdal B, Eds. New York, Plenum Press, 1994, p. 85–98 - PubMed
-
- McManus RM, Cunningham I, Watson A, Harker L, Finegood DT. Beta-cell function and visceral fat in lactating women with a history of gestational diabetes. Metabolism 2001;50:715–719 - PubMed
-
- Tigas S, Sunehag A, Haymond MW. Metabolic adaptation to feeding and fasting during lactation in humans. J Clin Endocrinol Metab 2002;87:302–307 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

