Impact of EMS bypass to endovascular capable hospitals: geospatial modeling analysis of the US STRATIS registry
- PMID: 32385089
- PMCID: PMC7569363
- DOI: 10.1136/neurintsurg-2019-015593
Impact of EMS bypass to endovascular capable hospitals: geospatial modeling analysis of the US STRATIS registry
Abstract
Background: Routing patients directly to endovascular capable centers (ECCs) would decrease time to mechanical thrombectomy (MT), but may delay intravenous thrombolysis (IVT).
Objective: To study the clinical outcomes of patients with a stroke transferred directly to ECCs compared with those transferred to ECCs from non-endovascular capable centers (nECCs).
Methods: Data from the STRATIS registry were analyzed to evaluate process and clinical outcomes under five routing policies: (1) transport to nearest nECC; (2) transport to STRATIS ECC over any distance or (3) within 20 miles; (4) transport to ideal ECC (iECC), over any distance or (5) within 20 miles.
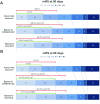
Results: Among 236 patients, 117 (49.6%) were transferred by ground, of whom 62 (53%) were transferred within 20 miles. Median MT start time was accelerated in all direct transport models. IVT start was prolonged with direct transport across all distances, but accelerated with direct transport to iECC ≤20 miles. With bypass limited to ≤20 miles, the median modeled EMS arrival to IVT interval decreased for both iECCs and ECCs (by 12 min and 6 min, respectively), and median EMS arrival to puncture time decreased by up to 94 min. In this cohort, no patient would have become ineligible for IVT. Bypass to iECC modeling under 20 miles showed a significant reduction in the level of disability at 3 months, with freedom from disability (modified Rankin Scale score 0-1) at 3 months increased by 12%.
Conclusions: Direct routing of patients with a large vessel occlusion to ECCs, especially when within 20 miles, may lead to better clinical outcomes by accelerating the start of MT without any delay of IVT.
Clinical trial registration number: http://www.clinicaltrials.gov. Unique identifier: NCT02239640.
Keywords: stroke; thrombectomy.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: NM-K and OZ serve as scientific consultants regarding trial design and conduct to Medtronic, who sponsored this study. MTF serves as a scientific consultant to Medtronic, Stryker, Balt, Viz.ai, NeurVana, and Genentech, and has received research funding from the National Institutes of Health (NIH), Stryker, Medtronic, Microvention, and Endophys. The University of California, Regents receives funding for Dr Jahan’s services as a scientific consultant regarding trial design and conduct to Medtronic/Covidien; RJ is an employee of the University of California, which holds a patent on retriever devices for stroke. The University of California, Regents receives funding for Dr Saver’s services as a scientific consultant regarding trial design and conduct to Covidien and Stryker; JLS is an employee of the University of California, which holds a patent on retriever devices for stroke. DL serves as an imaging core laboratory consultant for Cerenovus, Genentech, Medtronic, Stryker, and Vesalio.
Figures

References
-
- Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014;384:1929–35. 10.1016/S0140-6736(14)60584-5 - DOI - PMC - PubMed
