Increased Retinoic Acid Catabolism in Olfactory Sensory Neurons Activates Dormant Tissue-Specific Stem Cells and Accelerates Age-Related Metaplasia
- PMID: 32385093
- PMCID: PMC7244205
- DOI: 10.1523/JNEUROSCI.2468-19.2020
Increased Retinoic Acid Catabolism in Olfactory Sensory Neurons Activates Dormant Tissue-Specific Stem Cells and Accelerates Age-Related Metaplasia
Abstract
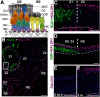
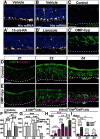
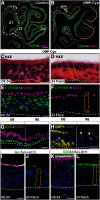
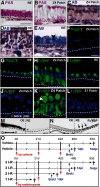
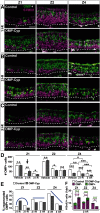
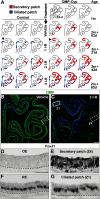
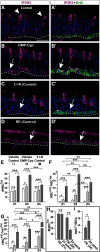
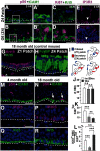
The cellular and molecular basis of metaplasia and declining neurogenesis in the aging olfactory epithelium (OE) remains unknown. The horizontal basal cell (HBC) is a dormant tissue-specific stem cell presumed to only be forced into self-renewal and differentiation by injury. Here we analyze male and female mice and show that HBCs also are activated with increasing age as well as non-cell-autonomously by increased expression of the retinoic acid-degrading enzyme CYP26B1. Activating stimuli induce HBCs throughout OE to acquire a rounded morphology and express IP3R3, which is an inositol-1,4,5-trisphosphate receptor constitutively expressed in stem cells of the adjacent respiratory epithelium. Odor/air stimulates CYP26B1 expression in olfactory sensory neurons mainly located in the dorsomedial OE, which is spatially inverse to ventrolateral constitutive expression of the retinoic acid-synthesizing enzyme (RALDH1) in supporting cells. In ventrolateral OE, HBCs express low p63 levels and preferentially differentiate instead of self-renewing when activated. When activated by chronic CYP26B1 expression, repeated injury, or old age, ventrolateral HBCs diminish in number and generate a novel type of metaplastic respiratory cell that is RALDH- and secretes a mucin-like mucus barrier protein (FcγBP). Conversely, in the dorsomedial OE, CYP26B1 inhibits injury-induced and age-related replacement of RALDH- supporting cells with RALDH1+ ciliated respiratory cells. Collectively, these results support the concept that inositol-1,4,5-trisphosphate type 3 receptor signaling in HBCs, together with altered retinoic acid metabolism within the niche, promote HBC lineage commitment toward two types of respiratory cells that will maintain epithelial barrier function once the capacity to regenerate OE cells ceases.SIGNIFICANCE STATEMENT Little is known about signals that activate dormant stem cells to self-renew and regenerate odor-detecting neurons and other olfactory cell types after loss due to injury, infection, or toxin exposure in the nose. It is also unknown why the stem cells do not prevent age-dependent decline of odor-detecting neurons. We show that (1) stem cells are kept inactive by the vitamin A derivative retinoic acid, which is synthesized and degraded locally by olfactory cells; (2) old age as well as repeated injuries activate the stem cells and exhaust their potential to produce olfactory cells; and (3) exhausted stem cells alter the local retinoic acid metabolism and maintain the epithelial tissue barrier by generating airway cells instead of olfactory cells.
Keywords: aging; inositol-1,4,5-triphosphate; metaplasia; olfactory epithelium; retinoic acid; stem cells.
Copyright © 2020 the authors.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
