Validation of serum neurofilaments as prognostic and potential pharmacodynamic biomarkers for ALS
- PMID: 32385188
- PMCID: PMC7371380
- DOI: 10.1212/WNL.0000000000009559
Validation of serum neurofilaments as prognostic and potential pharmacodynamic biomarkers for ALS
Abstract
Objective: To identify preferred neurofilament assays and clinically validate serum neurofilament light (NfL) and phosphorylated neurofilament heavy (pNfH) as prognostic and potential pharmacodynamic biomarkers relevant to amyotrophic lateral sclerosis (ALS) therapy development.
Methods: In this prospective, multicenter, longitudinal observational study of patients with ALS (n = 229), primary lateral sclerosis (n = 20), and progressive muscular atrophy (n = 11), biological specimens were collected, processed, and stored according to strict standard operating procedures (SOPs). Neurofilament assays were performed in a blinded manner by independent contract research organizations.
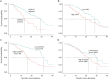
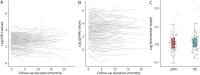
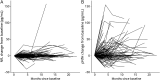
Results: For serum NfL and pNfH measured using the Simoa assay, there were no missing data (i.e., technical replicates below the lower limit of detection were not encountered). For the Iron Horse and Euroimmun pNfH assays, such missingness was encountered in ∼4% and ∼10% of serum samples, respectively. Mean coefficients of variation for NfL in serum and CSF were both ∼3%. Mean coefficients of variation for pNfH in serum and CSF were ∼4%-5% and ∼2%-3%, respectively, in all assays. Baseline serum NfL concentration, but not pNfH, predicted the future Revised ALS Functional Rating Scale (ALSFRS-R) slope and survival. Incorporation of baseline serum NfL into mixed effects models of ALSFRS-R slopes yields an estimated sample size saving of ∼8%. Depending on the method used to estimate effect size, use of serum NfL (and perhaps pNfH) as pharmacodynamic biomarkers, instead of the ALSFRS-R slope, yields significantly larger sample size savings.
Conclusions: Serum NfL may be considered a clinically validated prognostic biomarker for ALS. Serum NfL (and perhaps pNfH), quantified using the Simoa assay, has potential utility as a pharmacodynamic biomarker of treatment effect.
© 2020 American Academy of Neurology.
Figures
References
-
- Benatar M. ALS therapy development: challenges and opportunities. J Neuromuscul Dis 2016;3:S10.
-
- Verde F, Steinacker P, Weishaupt JH, et al. . Neurofilament light chain in serum for the diagnosis of amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2019;90:157–164. - PubMed
-
- Rossi D, Volanti P, Brambilla L, Colletti T, Spataro R, La Bella V. CSF neurofilament proteins as diagnostic and prognostic biomarkers for amyotrophic lateral sclerosis. J Neurol 2018;265:510–521. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
