Plasma membrane damage causes NLRP3 activation and pyroptosis during Mycobacterium tuberculosis infection
- PMID: 32385301
- PMCID: PMC7210277
- DOI: 10.1038/s41467-020-16143-6
Plasma membrane damage causes NLRP3 activation and pyroptosis during Mycobacterium tuberculosis infection
Abstract
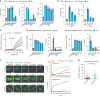
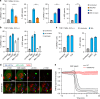
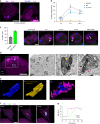
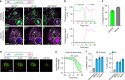
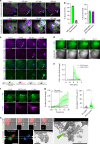
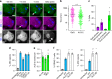
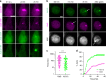
Mycobacterium tuberculosis is a global health problem in part as a result of extensive cytotoxicity caused by the infection. Here, we show how M. tuberculosis causes caspase-1/NLRP3/gasdermin D-mediated pyroptosis of human monocytes and macrophages. A type VII secretion system (ESX-1) mediated, contact-induced plasma membrane damage response occurs during phagocytosis of bacteria. Alternatively, this can occur from the cytosolic side of the plasma membrane after phagosomal rupture in infected macrophages. This damage causes K+ efflux and activation of NLRP3-dependent IL-1β release and pyroptosis, facilitating the spread of bacteria to neighbouring cells. A dynamic interplay of pyroptosis with ESCRT-mediated plasma membrane repair also occurs. This dual plasma membrane damage seems to be a common mechanism for NLRP3 activators that function through lysosomal damage.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- World Health Organization. Global tuberculosis report 2019 (WHO, 2019).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

