Heat shock protein 90-targeted photodynamic therapy enables treatment of subcutaneous and visceral tumors
- PMID: 32385408
- PMCID: PMC7210113
- DOI: 10.1038/s42003-020-0956-7
Heat shock protein 90-targeted photodynamic therapy enables treatment of subcutaneous and visceral tumors
Abstract
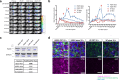
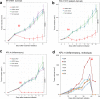
Photodynamic therapy (PDT) ablates malignancies by applying focused near-infrared (nIR) light onto a lesion of interest after systemic administration of a photosensitizer (PS); however, the accumulation of existing PS is not tumor-exclusive. We developed a tumor-localizing strategy for PDT, exploiting the high expression of heat shock protein 90 (Hsp90) in cancer cells to retain high concentrations of PS by tethering a small molecule Hsp90 inhibitor to a PS (verteporfin, VP) to create an Hsp90-targeted PS (HS201). HS201 accumulates to a greater extent than VP in breast cancer cells both in vitro and in vivo, resulting in increased treatment efficacy of HS201-PDT in various human breast cancer xenografts regardless of molecular and clinical subtypes. The therapeutic index achieved with Hsp90-targeted PDT would permit treatment not only of localized tumors, but also more diffusely infiltrating processes such as inflammatory breast cancer.
Conflict of interest statement
T.H. and P.H. have two issued patents pertinent to this work, US 10,112,947 B2 and US 9,738,643 B2, filed by Duke University. The remaining authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

