Distal γ-C(sp3 )-H Olefination of Ketone Derivatives and Free Carboxylic Acids
- PMID: 32385966
- PMCID: PMC7494175
- DOI: 10.1002/anie.202003271
Distal γ-C(sp3 )-H Olefination of Ketone Derivatives and Free Carboxylic Acids
Abstract
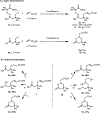
Reported herein is the distal γ-C(sp3 )-H olefination of ketone derivatives and free carboxylic acids. Fine tuning of a previously reported imino-acid directing group and using the ligand combination of a mono-N-protected amino acid (MPAA) and an electron-deficient 2-pyridone were critical for the γ-C(sp3 )-H olefination of ketone substrates. In addition, MPAAs enabled the γ-C(sp3 )-H olefination of free carboxylic acids to form diverse six-membered lactones. Besides alkyl carboxylic acids, benzylic C(sp3 )-H bonds also could be functionalized to form 3,4-dihydroisocoumarin structures in a single step from 2-methyl benzoic acid derivatives. The utility of these protocols was demonstrated in large scale reactions and diversification of the γ-C(sp3 )-H olefinated products.
Keywords: C(sp3)−H activation; amino acids; olefins; palladium; synthetic methods.
© 2020 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
Conflict of interest
The authors declare no conflict of interest.
Figures


References
-
- Baldwin JE, Jones RH, Nájera C, Yus M, Tetrahedron 1985, 41, 699;
- Constable AG, McDonald WS, Sawkins LC, Shaw BL, J. Chem. Soc., Chem. Commun 1978, 1061;
- Carr K, Sutherland JK, J. Chem. Soc., Chem. Commun 1984, 1227;
- Baldwin JE, Nájera C, Yus M, J. Chem. Soc., Chem. Commun 1985, 126.
-
- Desai LV, Hull KL, Sanford MS, J. Am. Chem. Soc 2004, 126, 9542; - PubMed
- Thu H-Y, Yu W-Y, Che C-M, J. Am. Chem. Soc 2006, 128, 9048; - PubMed
- Kang T, Kim Y, Lee D, Wang Z, Chang S, J. Am. Chem. Soc 2014, 136, 4141; - PubMed
- Gao P, Guo W, Xue J, Zhao Y, Yuan Y, Xia Y, Shi Z, J. Am. Chem. Soc 2015, 137, 12231; - PubMed
- Mu Y, Tan X, Zhang Y, Jing X, Shi Z, Org. Chem. Front 2016, 3, 380.
-
- Zhang F-L, Hong K, Li T-J, Park H, Yu J-Q, Science 2016, 351, 252; - PMC - PubMed
- Hong K, Park H, Yu J-Q, ACS Catal. 2017, 7, 6938; - PMC - PubMed
- Park H, Verma P, Hong K, Yu J-Q, Nat. Chem. 2018, 10, 755; - PMC - PubMed
- Yang K, Li Q, Liu Y, Li G, Ge H, J. Am. Chem. Soc 2016, 138, 12775; - PubMed
- Li B, Lawrence B, Li G, Ge H, Angew. Chem. Int. Ed 2020, 59, 3078; Angew. Chem. 2020, 132, 3102; - PubMed
- Dong C, Wu L, Yao J, Wei K, Org. Lett 2019, 21, 2085; - PubMed
- Wen F, Li Z, Adv. Synth. Catal 2019, 362, 133;
- St John-Campbell S, White AJP, Bull JA, Org. Lett 2020, 10.1021/acs.orglett.0c00124. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

