Reflection on modern methods: trial emulation in the presence of immortal-time bias. Assessing the benefit of major surgery for elderly lung cancer patients using observational data
- PMID: 32386426
- PMCID: PMC7823243
- DOI: 10.1093/ije/dyaa057
Reflection on modern methods: trial emulation in the presence of immortal-time bias. Assessing the benefit of major surgery for elderly lung cancer patients using observational data
Abstract
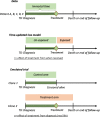
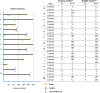
Acquiring real-world evidence is crucial to support health policy, but observational studies are prone to serious biases. An approach was recently proposed to overcome confounding and immortal-time biases within the emulated trial framework. This tutorial provides a step-by-step description of the design and analysis of emulated trials, as well as R and Stata code, to facilitate its use in practice. The steps consist in: (i) specifying the target trial and inclusion criteria; (ii) cloning patients; (iii) defining censoring and survival times; (iv) estimating the weights to account for informative censoring introduced by design; and (v) analysing these data. These steps are illustrated with observational data to assess the benefit of surgery among 70-89-year-old patients diagnosed with early-stage lung cancer. Because of the severe unbalance of the patient characteristics between treatment arms (surgery yes/no), a naïve Kaplan-Meier survival analysis of the initial cohort severely overestimated the benefit of surgery on 1-year survival (22% difference), as did a survival analysis of the cloned dataset when informative censoring was ignored (17% difference). By contrast, the estimated weights adequately removed the covariate imbalance. The weighted analysis still showed evidence of a benefit, though smaller (11% difference), of surgery among older lung cancer patients on 1-year survival. Complementing the CERBOT tool, this tutorial explains how to proceed to conduct emulated trials using observational data in the presence of immortal-time bias. The strength of this approach is its transparency and its principles that are easily understandable by non-specialists.
Keywords: Observational data; elderly; immortal-time bias; inverse-probability-of-censoring weighting; lung cancer; trial emulation.
© The Author(s) 2020. Published by Oxford University Press on behalf of the International Epidemiological Association.
Figures

Comment in
-
Versatility of the clone-censor-weight approach: response to "trial emulation in the presence of immortal-time bias".Int J Epidemiol. 2021 May 17;50(2):694-695. doi: 10.1093/ije/dyaa223. Int J Epidemiol. 2021. PMID: 33349843 No abstract available.
-
Reply to: Versatility of the clone-censor-weight approach: response to ''trial emulation in the presence of immortal-time bias''.Int J Epidemiol. 2021 May 17;50(2):696. doi: 10.1093/ije/dyaa225. Int J Epidemiol. 2021. PMID: 33524137 Free PMC article. No abstract available.
References
-
- Belot A, Fowler H, Njagi EN. et al. Association between age, deprivation and specific comorbid conditions and the receipt of major surgery in patients with non-small cell lung cancer in England: A population-based study. Thorax 2019;74:51–59. - PubMed
-
- Johnstone DW, Byhardt RW, Ettinger D, Scott CB.. Phase III study comparing chemotherapy and radiotherapy with preoperative chemotherapy and surgical resection in patients with non-small-cell lung cancer with spread to mediastinal lymph nodes (N2); final report of RTOG 89-01. Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 2002;54:365–69. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

