Frontline Science: Kindlin-3 is essential for patrolling and phagocytosis functions of nonclassical monocytes during metastatic cancer surveillance
- PMID: 32386455
- PMCID: PMC7986576
- DOI: 10.1002/JLB.4HI0420-098R
Frontline Science: Kindlin-3 is essential for patrolling and phagocytosis functions of nonclassical monocytes during metastatic cancer surveillance
Abstract
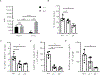
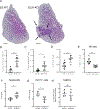
Nonclassical monocytes maintain vascular homeostasis by patrolling the vascular endothelium, responding to inflammatory signals, and scavenging cellular debris. Nonclassical monocytes also prevent metastatic tumor cells from seeding new tissues, but whether the patrolling function of nonclassical monocytes is required for this process is unknown. To answer this question, we utilized an inducible-knockout mouse that exhibits loss of the integrin-adaptor protein Kindlin-3 specifically in nonclassical monocytes. We show that Kindlin-3-deficient nonclassical monocytes are unable to patrol the vascular endothelium in either the lungs or periphery. We also find that Kindlin-3-deficient nonclassical monocytes cannot firmly adhere to, and instead "slip" along, the vascular endothelium. Loss of patrolling activity by nonclassical monocytes was phenocopied by ablation of LFA-1, an integrin-binding partner of Kindlin-3. When B16F10 murine melanoma tumor cells were introduced into Kindlin-3-deficient mice, nonclassical monocytes showed defective patrolling towards tumor cells and failure to ingest tumor particles in vivo. Consequently, we observed a significant, 4-fold increase in lung tumor metastases in mice possessing Kindlin-3-deficient nonclassical monocytes. Thus, we conclude that the patrolling function of nonclassical monocytes is mediated by Kindlin-3 and essential for these cells to maintain vascular endothelial homeostasis and prevent tumor metastasis to the lung.
Keywords: endothelium; integrins; lungs; metastasis; nonclassical monocytes; patrolling.
©2020 Society for Leukocyte Biology.
Conflict of interest statement
CONFLICTS OF INTEREST DISCLOSURE
The authors declare no potential conflicts of interest.
Figures

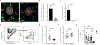
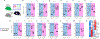
Comment in
-
Kindlin-3 gives patrolling monocytes a strong grip.J Leukoc Biol. 2020 Jun;107(6):879-881. doi: 10.1002/JLB.3CE0320-168. J Leukoc Biol. 2020. PMID: 32533639 Free PMC article.
References
-
- Nguyen DX, Bos PD, Massague J (2009) Metastasis: from dissemination to organ-specific colonization. Nature reviews 9, 274–84. - PubMed
-
- Weidle UH, Birzele F, Kollmorgen G, Ruger R (2016) Molecular Basis of Lung Tropism of Metastasis. Cancer Genomics Proteomics 13, 129–39. - PubMed
-
- Sagiv JY, Michaeli J, Assi S, Mishalian I, Kisos H, Levy L, Damti P, Lumbroso D, Polyansky L, Sionov RV, Ariel A, Hovav AH, Henke E, Fridlender ZG, Granot Z (2015) Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep 10, 562–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

