Single-Cell Analyses Reveal Megakaryocyte-Biased Hematopoiesis in Myelofibrosis and Identify Mutant Clone-Specific Targets
- PMID: 32386542
- PMCID: PMC7217381
- DOI: 10.1016/j.molcel.2020.04.008
Single-Cell Analyses Reveal Megakaryocyte-Biased Hematopoiesis in Myelofibrosis and Identify Mutant Clone-Specific Targets
Abstract
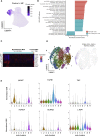
Myelofibrosis is a severe myeloproliferative neoplasm characterized by increased numbers of abnormal bone marrow megakaryocytes that induce fibrosis, destroying the hematopoietic microenvironment. To determine the cellular and molecular basis for aberrant megakaryopoiesis in myelofibrosis, we performed single-cell transcriptome profiling of 135,929 CD34+ lineage- hematopoietic stem and progenitor cells (HSPCs), single-cell proteomics, genomics, and functional assays. We identified a bias toward megakaryocyte differentiation apparent from early multipotent stem cells in myelofibrosis and associated aberrant molecular signatures. A sub-fraction of myelofibrosis megakaryocyte progenitors (MkPs) are transcriptionally similar to healthy-donor MkPs, but the majority are disease specific, with distinct populations expressing fibrosis- and proliferation-associated genes. Mutant-clone HSPCs have increased expression of megakaryocyte-associated genes compared to wild-type HSPCs, and we provide early validation of G6B as a potential immunotherapy target. Our study paves the way for selective targeting of the myelofibrosis clone and illustrates the power of single-cell multi-omics to discover tumor-specific therapeutic targets and mediators of tissue fibrosis.
Keywords: G6B; TARGET-seq; bone marrow; fibrosis; immunotherapy; megakaryopoiesis; myeloproliferative neoplasm; platelets; single-cell multi-omics.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no relevant competing interests.
Figures



References
-
- Adolfsson J., Månsson R., Buza-Vidas N., Hultquist A., Liuba K., Jensen C.T., Bryder D., Yang L., Borge O.J., Thoren L.A. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. - PubMed
-
- Akashi K., Traver D., Miyamoto T., Weissman I.L. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. - PubMed
-
- Allen R.J., Porte J., Braybrooke R., Flores C., Fingerlin T.E., Oldham J.M., Guillen-Guio B., Ma S.F., Okamoto T., John A.E. Genetic variants associated with susceptibility to idiopathic pulmonary fibrosis in people of European ancestry: a genome-wide association study. Lancet Respir. Med. 2017;5:869–880. - PMC - PubMed
-
- Arber D.A., Orazi A., Hasserjian R., Thiele J., Borowitz M.J., Le Beau M.M., Bloomfield C.D., Cazzola M., Vardiman J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MR/M00919X/1/MRC_/Medical Research Council/United Kingdom
- G84/6443/MRC_/Medical Research Council/United Kingdom
- 28051/CRUK_/Cancer Research UK/United Kingdom
- 26988/CRUK_/Cancer Research UK/United Kingdom
- MR/S001190/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_00016/15/MRC_/Medical Research Council/United Kingdom
- MC_UU_12009/16/MRC_/Medical Research Council/United Kingdom
- 24593/CRUK_/Cancer Research UK/United Kingdom
- 27723/CRUK_/Cancer Research UK/United Kingdom
- MR/L006340/1/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- 27125/CRUK_/Cancer Research UK/United Kingdom
- 29034/CRUK_/Cancer Research UK/United Kingdom
- 30721/CRUK_/Cancer Research UK/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

