Hematopoietic Stem and Progenitor Cells Exhibit Stage-Specific Translational Programs via mTOR- and CDK1-Dependent Mechanisms
- PMID: 32386556
- PMCID: PMC9435590
- DOI: 10.1016/j.stem.2019.12.006
Hematopoietic Stem and Progenitor Cells Exhibit Stage-Specific Translational Programs via mTOR- and CDK1-Dependent Mechanisms
Abstract
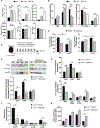
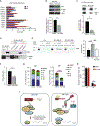
Hematopoietic stem cells (HSCs) require highly regulated rates of protein synthesis, but it is unclear if they or lineage-committed progenitors preferentially recruit transcripts to translating ribosomes. We utilized polysome profiling, RNA sequencing, and whole-proteomic approaches to examine the translatome in LSK (Lin-Sca-1+c-Kit+) and myeloid progenitor (MP; Lin-Sca-1-c-Kit+) cells. Our studies show that LSKs exhibit low global translation but high translational efficiencies (TEs) of mRNAs required for HSC maintenance. In contrast, MPs activate translation in an mTOR-independent manner due, at least in part, to proteasomal degradation of mTOR by the E3 ubiquitin ligase c-Cbl. In the near absence of mTOR, CDK1 activates eIF4E-dependent translation in MPs through phosphorylation of 4E-BP1. Aberrant activation of mTOR expression and signaling in c-Cbl-deficient MPs results in increased mature myeloid lineage output. Overall, our data demonstrate that hematopoietic stem and progenitor cells (HSPCs) undergo translational reprogramming mediated by previously uncharacterized mechanisms of translational regulation.
Keywords: hematopoiesis; mTOR; myeloid progenitors; polysome; proteomics; ribosome; stem cells; transcriptome; translation; ubiquitination.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures


References
-
- Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, and Sabatini DM (2006). Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell 11, 859–871. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

