Blockade of Oncogenic NOTCH1 with the SERCA Inhibitor CAD204520 in T Cell Acute Lymphoblastic Leukemia
- PMID: 32386594
- PMCID: PMC7305996
- DOI: 10.1016/j.chembiol.2020.04.002
Blockade of Oncogenic NOTCH1 with the SERCA Inhibitor CAD204520 in T Cell Acute Lymphoblastic Leukemia
Abstract
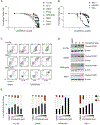
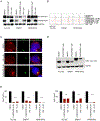
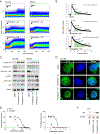
The identification of SERCA (sarco/endoplasmic reticulum calcium ATPase) as a target for modulating gain-of-function NOTCH1 mutations in Notch-dependent cancers has spurred the development of this compound class for cancer therapeutics. Despite the innate toxicity challenge associated with SERCA inhibition, we identified CAD204520, a small molecule with better drug-like properties and reduced off-target Ca2+ toxicity compared with the SERCA inhibitor thapsigargin. In this work, we describe the properties and complex structure of CAD204520 and show that CAD204520 preferentially targets mutated over wild-type NOTCH1 proteins in T cell acute lymphoblastic leukemia (T-ALL) and mantle cell lymphoma (MCL). Uniquely among SERCA inhibitors, CAD204520 suppresses NOTCH1-mutated leukemic cells in a T-ALL xenografted model without causing cardiac toxicity. This study supports the development of SERCA inhibitors for Notch-dependent cancers and extends their application to cases with isolated mutations in the PEST degradation domain of NOTCH1, such as MCL or chronic lymphocytic leukemia (CLL).
Keywords: CAD204520; NOTCH1; NOTCH1 mutation; P-type ATPases screening; PEST mutation; SERCA; T cell acute lymphoblastic leukemia (T-ALL); crystal structure; mantle cell lymphoma (MCL); thapsigargin.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Author Contributions Conceptualization and Design, M.M., A.G., W.D.-B., A.-M.L.W., M.B., and G.R.; Methodology, all authors; Resources, all authors; Investigation, all authors; Formal Analysis, all authors; Writing – Original Draft, W.D.-B., A.-M.L.W., M.M., M.B., and G.R.; Writing – Review & Editing, W.D.-B., A.-M.L.W., M.M., M.B., and G.R.; Data Curation, W.D.-B., A.-M.L.W., P.S., R.L.S., C.M., F.A., K.S., M.B., and G.R. Funding Acquisition, P.S., C.M., R.L.S., K.S., M.M., and G.R.; Project Administration, M.M. and G.R.; Supervision, G.R. Declaration of Interests W.D.-B. and A.-M.L.W. are CaDo Biotechnology IvS employees. The compounds studied are part of a patent application wholly owned by CaDo Biotechnology IvS, Denmark. K.S. has previously consulted for Novartis and Rigel Pharmaceuticals and currently receives grant funding from Novartis on topics unrelated to this manuscript.
Figures




References
-
- Andersen JP, Lassen K, and Moller JV (1985). Changes in Ca2+ affinity related to conformational transitions in the phosphorylated state of soluble monomeric Ca2+-ATPase from sarcoplasmic reticulum. J Biol Chem 260, 371–380. - PubMed
-
- Arbabian A, Brouland JP, Gelebart P, Kovacs T, Bobe R, Enouf J, and Papp B (2011). Endoplasmic reticulum calcium pumps and cancer. Biofactors 37, 139–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

