Targeted Degradation of SLC Transporters Reveals Amenability of Multi-Pass Transmembrane Proteins to Ligand-Induced Proteolysis
- PMID: 32386596
- PMCID: PMC7303955
- DOI: 10.1016/j.chembiol.2020.04.003
Targeted Degradation of SLC Transporters Reveals Amenability of Multi-Pass Transmembrane Proteins to Ligand-Induced Proteolysis
Abstract
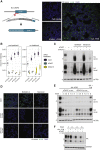
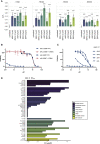
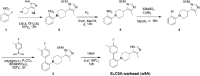
With more than 450 members, the solute carrier (SLC) group of proteins represents the largest class of transporters encoded in the human genome. Their several-pass transmembrane domain structure and hydrophobicity contribute to the orphan status of many SLCs, devoid of known cargos or chemical inhibitors. We report that SLC proteins belonging to different families and subcellular compartments are amenable to induced degradation by heterobifunctional ligands. Engineering endogenous alleles via the degradation tag (dTAG) technology enabled chemical control of abundance of the transporter protein, SLC38A2. Moreover, we report the design of d9A-2, a chimeric compound engaging several members of the SLC9 family and leading to their degradation. d9A-2 impairs cellular pH homeostasis and promotes cell death in a range of cancer cell lines. These findings open the era of SLC-targeting chimeric degraders and demonstrate potential access of multi-pass transmembrane proteins of different subcellular localizations to the chemically exploitable degradation machinery.
Keywords: E3 ligase; SLC38A2; SLC9A1; degrader; multi-pass transmembrane protein; proteasome; proteolysis targeting chimera (PROTAC); solute carrier; targeted degradation; transporter.
Copyright © 2020 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Interests A.B., G.E.W., and G.S.-F. are co-authors of a patent application and co-founders of a company related to SLCs. G.S.-F. is the Academic Project Coordinator of the IMI grant RESOLUTE in partnership with Pfizer, Novartis, Bayer, Sanofi, Boehringer Ingelheim, and Vifor Pharma. The G.S.-F. laboratory receives funds from Pfizer and Boehringer Ingelheim.
Figures





References
-
- Amith S.R., Fliegel L. Regulation of the Na+/H+ exchanger (NHE1) in breast cancer metastasis. Cancer Res. 2013;73:1259–1264. - PubMed
-
- Atwal K.S., O’Neil S.V., Ahmad S., Doweyko L., Kirby M., Dorso C.R., Chandrasena G., Chen B.-C., Zhao R., Zahler R. Synthesis and biological activity of 5-aryl-4-(4-(5-methyl-1H-imidazol-4-yl)piperidin-1-yl)pyrimidine analogs as potent, highly selective, and orally bioavailable NHE-1 inhibitors. Bioorg. Med. Chem. Lett. 2006;16:4796–4799. - PubMed
-
- Bai X., Moraes T.F., Reithmeier R.A.F. Structural biology of solute carrier (SLC) membrane transport proteins. Mol. Membr. Biol. 2017;34:1–32. - PubMed
-
- Barkai N., Leibler S. Robustness in simple biochemical networks. Nature. 1997;387:913–917. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

