Influence of immune aging on vaccine responses
- PMID: 32386655
- PMCID: PMC7198995
- DOI: 10.1016/j.jaci.2020.03.017
Influence of immune aging on vaccine responses
Abstract
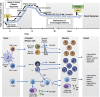
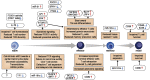
Impaired vaccine responses in older individuals are associated with alterations in both the quantity and quality of the T-cell compartment with age. As reviewed herein, the T-cell response to vaccination requires a fine balance between the generation of inflammatory effector T cells versus follicular helper T (TFH) cells that mediate high-affinity antibody production in tandem with the induction of long-lived memory cells for effective recall immunity. During aging, we find that this balance is tipped where T cells favor short-lived effector but not memory or TFH responses. Consistently, vaccine-induced antibodies commonly display a lower protective capacity. Mechanistically, multiple, potentially targetable, changes in T cells have been identified that contribute to these age-related defects, including posttranscription regulation, T-cell receptor signaling, and metabolic function. Although research into the induction of tissue-specific immunity by vaccines and with age is still limited, current mechanistic insights provide a framework for improved design of age-specific vaccination strategies that require further evaluation in a clinical setting.
Keywords: T cells; T-cell receptor; Vaccination; age; antibody; recall response.
Copyright © 2020 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Nikolich-Zugich J. The twilight of immunity: emerging concepts in aging of the immune system. Nat Immunol. 2018;19:10–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

