Raltegravir versus efavirenz in antiretroviral-naive pregnant women living with HIV (NICHD P1081): an open-label, randomised, controlled, phase 4 trial
- PMID: 32386720
- PMCID: PMC7323582
- DOI: 10.1016/S2352-3018(20)30038-2
Raltegravir versus efavirenz in antiretroviral-naive pregnant women living with HIV (NICHD P1081): an open-label, randomised, controlled, phase 4 trial
Erratum in
-
Correction to Lancet HIV 2020; 7: e322-31.Lancet HIV. 2020 Dec;7(12):e803. doi: 10.1016/S2352-3018(20)30168-5. Epub 2020 Jun 9. Lancet HIV. 2020. PMID: 32531263 No abstract available.
Abstract
Background: Although antiretroviral regimens containing integrase inhibitors rapidly suppress HIV viral load in non-pregnant adults, few published data from randomised controlled trials have compared the safety and efficacy of any integrase inhibitor to efavirenz when initiated during pregnancy. We compared safety and efficacy of antiretroviral therapy with either raltegravir or efavirenz in late pregnancy.
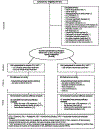
Methods: An open-label, randomised controlled trial was done at 19 hospitals and clinics in Argentina, Brazil, South Africa, Tanzania, Thailand, and the USA. Antiretroviral-naive pregnant women (20-<37 weeks gestation) living with HIV were assigned to antiretroviral regimens containing either raltegravir (400 mg twice daily) or efavirenz (600 mg each night) plus lamivudine 150 mg and zidovudine 300 mg twice daily (or approved alternative backbone regimen), using a web-based, permuted-block randomisation stratified by gestational age and backbone regimen. The primary efficacy outcome was plasma HIV viral load below 200 copies per mL at (or near) delivery. The primary efficacy analysis included all women with a viral load measurement at (or near) delivery who had viral load of at least 200 copies per mL before treatment and no genotypic resistance to any study drugs; secondary analyses eliminated these exclusion criteria. The primary safety analyses included all women who received study drug, and their infants. This trial is registered with Clinicaltrials.gov, number NCT01618305.
Findings: From Sep 5, 2013, to Dec 11, 2018, 408 women were enrolled (206 raltegravir, 202 efavirenz) and 394 delivered on-study (200 raltegravir, 194 efavirenz); 307 were included in the primary efficacy analysis (153 raltegravir, 154 efavirenz). 144 (94%) women in the raltegravir group and 129 (84%) in the efavirenz group met the primary efficacy outcome (absolute difference 10%, 95% CI 3-18; p=0·0015); the difference primarily occurred among women enrolling later in pregnancy (interaction p=0·040). Frequencies of severe or life-threatening adverse events were similar among mothers (30% in each group; 61 raltegravir, 59 efavirenz) and infants (25% in each group; 50 raltegravir, 48 efavirenz), with no treatment-related deaths.
Interpretation: Our findings support major guidelines. The integrase inhibitor dolutegravir is currently a preferred regimen for the prevention of perinatal HIV transmission with raltegravir recommended as a preferred or alternative integrase inhibitor for pregnant women living with HIV.
Funding: Eunice Kennedy Shriver National Institute of Child Health and Human Development and National Institute of Allergy and Infectious Diseases.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures

Comment in
-
Enrolling pregnant women with HIV into clinical trials.Lancet HIV. 2020 May;7(5):e302-e303. doi: 10.1016/S2352-3018(20)30078-3. Lancet HIV. 2020. PMID: 32386716 No abstract available.
References
-
- UNAIDS. Fact Sheet - Global AIDS Update 2019. Geneva: UNAIDS; 2019.
-
- AIDSinfo. Recommendations for the Use of Antiretroviral Drugs in Pregnant Women with HIV Infection and Interventions to Reduce Perinatal HIV Transmission in the United States. Washington, DC: US Department of Health and Human Services; 2019.
-
- Mandelbrot L, Tubiana R, Le Chenadec J, et al. No perinatal HIV-1 transmission from women with effective antiretroviral therapy starting before conception. Clin Infect Dis 2015; 61(11): 1715–25. - PubMed
-
- Lennox JL, DeJesus E, Lazzarin A, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet 2009; 374(9692): 796–806. - PubMed
-
- WHO. Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV: interim guidelines Supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Geneva: World Health Organization; 2018.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

