Dolutegravir versus efavirenz in women starting HIV therapy in late pregnancy (DolPHIN-2): an open-label, randomised controlled trial
- PMID: 32386721
- PMCID: PMC10877544
- DOI: 10.1016/S2352-3018(20)30050-3
Dolutegravir versus efavirenz in women starting HIV therapy in late pregnancy (DolPHIN-2): an open-label, randomised controlled trial
Abstract
Background: Late initiation of HIV antiretroviral therapy (ART) in pregnancy is associated with not achieving viral suppression before giving birth and increased mother-to-child transmission of HIV. We aimed to investigate virological suppression before giving birth with dolutegravir compared with efavirenz, when initiated during the third trimester.
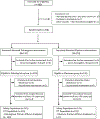
Methods: In this randomised, open-label trial, DolPHIN-2, we recruited pregnant women in South Africa and Uganda aged at least 18 years, with untreated but confirmed HIV infection and an estimated gestation of at least 28 weeks, initiating ART in third trimester. Participants were randomly assigned (1:1) to dolutegravir-based or efavirenz-based therapy. HIV viral load was measured 7 days and 28 days after antiretroviral initiation, at 36 weeks' gestation, and at the post-partum visit (0-14 days post partum). The primary efficacy outcome was a viral load of less than 50 copies per mL at the first post-partum visit, and the primary safety outcome was the occurrence of drug-related adverse events in mothers and infants until the post-partum visit. Longer-term follow-up of mothers and infants continues. This study is registered with ClinicalTrials.gov, NCT03249181.
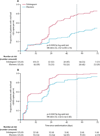
Findings: Between Jan 23, and Aug 15, 2018, we randomly assigned 268 mothers to dolutegravir (135) or efavirenz (133). All mothers and their infants were included in the safety analysis, and 250 mothers (125 in the dolutegravir group, 125 in the efavirenz group) and their infants in efficacy analyses, by intention-to-treat analyses. The median duration of maternal therapy at birth was 55 days (IQR 33-77). 89 (74%) of 120 in the dolutegravir group had viral loads less than 50 copies per mL, compared with 50 (43%) of 117 in the efavirenz group (risk ratio 1·64, 95% CI 1·31-2·06). 30 (22%) of 137 mothers in the dolutegravir group reported serious adverse events compared with 14 (11%) of 131 in the efavirenz group (p=0·013), particularly surrounding pregnancy and puerperium. We found no differences in births less than 37 weeks and less than 34 weeks gestation (16·4% vs 3·3%, across both groups). Three stillbirths in the dolutegravir group and one in the efavirenz group were considered unrelated to treatment. Three infant HIV infections were detected, all in the dolutegravir group, and were considered likely to be in-utero transmissions.
Interpretation: Our data support the revision to WHO guidelines recommending the transition to dolutegravir in first-line ART for all adults, regardless of pregnancy or child-bearing potential.
Funding: Unitaid.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
Opportunities and limits for dolutegravir in late pregnancy.Lancet HIV. 2020 May;7(5):e303-e304. doi: 10.1016/S2352-3018(20)30071-0. Lancet HIV. 2020. PMID: 32386717 No abstract available.
Similar articles
-
72 weeks post-partum follow-up of dolutegravir versus efavirenz initiated in late pregnancy (DolPHIN-2): an open-label, randomised controlled study.Lancet HIV. 2022 Aug;9(8):e534-e543. doi: 10.1016/S2352-3018(22)00173-4. Lancet HIV. 2022. PMID: 35905752 Clinical Trial.
-
Efficacy and safety of dolutegravir with emtricitabine and tenofovir alafenamide fumarate or tenofovir disoproxil fumarate, and efavirenz, emtricitabine, and tenofovir disoproxil fumarate HIV antiretroviral therapy regimens started in pregnancy (IMPAACT 2010/VESTED): a multicentre, open-label, randomised, controlled, phase 3 trial.Lancet. 2021 Apr 3;397(10281):1276-1292. doi: 10.1016/S0140-6736(21)00314-7. Lancet. 2021. PMID: 33812487 Free PMC article. Clinical Trial.
-
Safety and pharmacokinetics of dolutegravir in pregnant mothers with HIV infection and their neonates: A randomised trial (DolPHIN-1 study).PLoS Med. 2019 Sep 20;16(9):e1002895. doi: 10.1371/journal.pmed.1002895. eCollection 2019 Sep. PLoS Med. 2019. PMID: 31539371 Free PMC article. Clinical Trial.
-
Comparative efficacy and safety of dolutegravir relative to common core agents in treatment-naïve patients infected with HIV-1: a systematic review and network meta-analysis.BMC Infect Dis. 2019 May 30;19(1):484. doi: 10.1186/s12879-019-3975-6. BMC Infect Dis. 2019. PMID: 31146698 Free PMC article.
-
Neurotoxicities in the treatment of HIV between dolutegravir, rilpivirine and dolutegravir/rilpivirine: a meta-analysis.Sex Transm Infect. 2021 Jun;97(4):261-267. doi: 10.1136/sextrans-2020-054821. Epub 2021 Mar 29. Sex Transm Infect. 2021. PMID: 33782144
Cited by
-
Preventing perinatal HIV acquisition; current gaps and future perspectives.Curr Opin HIV AIDS. 2024 Nov 1;19(6):293-304. doi: 10.1097/COH.0000000000000881. Epub 2024 Aug 21. Curr Opin HIV AIDS. 2024. PMID: 39196368 Free PMC article. Review.
-
Antiretroviral Therapy in Pregnancy: A 2023 Review of the Literature.Curr HIV/AIDS Rep. 2024 Feb;21(1):1-10. doi: 10.1007/s11904-024-00688-y. Epub 2024 Jan 26. Curr HIV/AIDS Rep. 2024. PMID: 38277098 Free PMC article. Review.
-
Ending the evidence gap for pregnancy, HIV and co-infections: ethics guidance from the PHASES project.J Int AIDS Soc. 2021 Dec;24(12):e25846. doi: 10.1002/jia2.25846. J Int AIDS Soc. 2021. PMID: 34910846 Free PMC article.
-
Recent Antiretroviral Therapy Initiation Is Associated With Increased Mortality Risk in HIV-associated Cryptococcal Meningitis: An Analysis of Clinical Trial Data From Africa.Clin Infect Dis. 2025 Aug 1;81(1):75-83. doi: 10.1093/cid/ciae586. Clin Infect Dis. 2025. PMID: 40608053 Free PMC article.
-
Clinical and population-based study design considerations to accelerate the investigation of new antiretrovirals during pregnancy.J Int AIDS Soc. 2022 Jul;25 Suppl 2(Suppl 2):e25917. doi: 10.1002/jia2.25917. J Int AIDS Soc. 2022. PMID: 35851758 Free PMC article.
References
-
- Statistics UBO. Uganda Demographic and Health Survey 2016. Rockville, Maryland, USA: 2018.
-
- Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. The New England Journal of Medicine. 2013;369(19):1807–18. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

