Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients
- PMID: 32387320
- PMCID: PMC7202806
- DOI: 10.1016/j.medmal.2020.05.001
Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients
Abstract
Introduction: No therapy has yet proven effective in COVID-19. Tocilizumab (TCZ) in patients with severe COVID-19 could be an effective treatment.
Method: We conducted a retrospective case-control study in the Nord Franche-Comté Hospital, France. We compared the outcome of patients treated with TCZ and patients without TCZ considering a combined primary endpoint: death and/or ICU admissions.
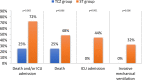
Results: Patients with TCZ (n=20) had a higher Charlson comorbidity index (5.3 [±2.4] vs 3.4 [±2.6], P=0.014), presented with more severe forms (higher level of oxygen therapy at 13L/min vs 6L/min, P<0.001), and had poorer biological findings (severe lymphopenia: 676/mm3 vs 914/mm3, P=0.037 and higher CRP level: 158mg/L vs 105mg/L, P=0.017) than patients without TCZ (n=25). However, death and/or ICU admissions were higher in patients without TCZ than in the TCZ group (72% vs 25%, P=0.002).
Conclusion: Despite the small sample size and retrospective nature of the work, this result strongly suggests that TCZ may reduce the number of ICU admissions and/or mortality in patients with severe SARS-CoV-2 pneumonia.
Keywords: COVID-19; Intensive care unit; Mortality; SARS-CoV-2; Tocilizumab.
Copyright © 2020 Elsevier Masson SAS. All rights reserved.
Figures
References
-
- Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for Coronavirus Disease 2019 (COVID-19): a review. JAMA. 2020 - PubMed
-
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med [Internet] 2020 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7164771/ [cité 26 avr 2020] - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous