The Accumulation of Tau in Postsynaptic Structures: A Common Feature in Multiple Neurodegenerative Diseases?
- PMID: 32389059
- PMCID: PMC8447404
- DOI: 10.1177/1073858420916696
The Accumulation of Tau in Postsynaptic Structures: A Common Feature in Multiple Neurodegenerative Diseases?
Abstract
Increasingly, research suggests that neurodegenerative diseases and dementias are caused not by unique, solitary cellular mechanisms, but by multiple contributory mechanisms manifesting as heterogeneous clinical presentations. However, diverse neurodegenerative diseases also share common pathological hallmarks and cellular mechanisms. One such mechanism involves the redistribution of the microtubule associated protein tau from the axon into the somatodendritic compartment of neurons, followed by the mislocalization of tau into dendritic spines, resulting in postsynaptic functional deficits. Here we review various signaling pathways that trigger the redistribution of tau to the cell body and dendritic tree, and its mislocalization to dendritic spines. The convergence of multiple pathways in different disease models onto this final common pathway suggests that it may be an attractive pathway to target for developing new treatments for neurodegenerative diseases.
Keywords: AMPA receptors; Alzheimer’s disease; FTDP-17; Huntington’s disease; MAPT; Parkinson’s disease; dendritic spines; neuronal polarity; synaptic plasticity; tau.
Conflict of interest statement
Figures


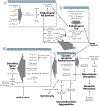
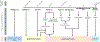


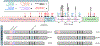
References
-
- Atlas R, Behar L, Sapoznik S, and Ginzburg I. 2007. Dynamic association with polysomes during P19 neuronal differentiation and an untranslated-region-dependent translation regulation of the tau mRNA by the tau mRNA-associated proteins IMP1, HuD, and G3BP1. J Neurosci Res. 85(1):173–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

