Target Product Profiles for medical tests: a systematic review of current methods
- PMID: 32389127
- PMCID: PMC7212678
- DOI: 10.1186/s12916-020-01582-1
Target Product Profiles for medical tests: a systematic review of current methods
Abstract
Background: A Target Product Profile (TPP) outlines the necessary characteristics of an innovative product to address an unmet clinical need. TPPs could be used to better guide manufacturers in the development of 'fit for purpose' tests, thus increasing the likelihood that novel tests will progress from bench to bedside. However, there is currently no guidance on how to produce a TPP specifically for medical tests.
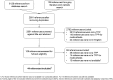
Methods: A systematic review was conducted to summarise the methods currently used to develop TPPs for medical tests, the sources used to inform these recommendations and the test characteristics for which targets are made. Database and website searches were conducted in November 2018. TPPs written in English for any medical test were included. Based on an existing framework, test characteristics were clustered into commonly recognised themes.
Results: Forty-four TPPs were identified, all of which focused on diagnostic tests for infectious diseases. Three core decision-making phases for developing TPPs were identified: scoping, drafting and consensus-building. Consultations with experts and the literature mostly informed the scoping and drafting of TPPs. All TPPs provided information on unmet clinical need and desirable analytical performance, and the majority specified clinical validity characteristics. Few TPPs described specifications for clinical utility, and none included cost-effectiveness.
Conclusions: We have identified a commonly used framework that could be beneficial for anyone interested in drafting a TPP for a medical test. Currently, key outcomes such as utility and cost-effectiveness are largely overlooked within TPPs though and we foresee this as an area for further improvement.
Keywords: Diagnostic; Medical test; Quality by design; TPP; Target Product Profile; Test characteristic.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Wang P, Kricka L. Current and emerging trends in point-of-care technology and strategies for clinical validation and implementation. Clin Chem. 2018. 10.1373/clinchem.2018.287052. - PubMed
-
- Horvath A, Lord S, St John A, Sandberg S, Cobbaert C, Lorenz S, et al. From biomarkers to medical tests: the changing landscape of test evaluation. Clin Chim Acta. 2014. 10.1016/j.cca.2013.09.018. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

