Dynamic profile for the detection of anti-SARS-CoV-2 antibodies using four immunochromatographic assays
- PMID: 32389784
- PMCID: PMC7204722
- DOI: 10.1016/j.jinf.2020.04.033
Dynamic profile for the detection of anti-SARS-CoV-2 antibodies using four immunochromatographic assays
Abstract
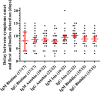
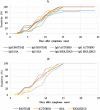
In order to fight the SARS-CoV-2 pandemic infection, there is a growing need and demand for diagnostic tools that are complementary and different from the RT-PCR currently in use. Multiple serological tests are or will be very soon available but need to be evaluated and validated. We have thus tested 4 immunochromatographic tests for the detection of antibodies to SARS-CoV-2. In addition, we assessed the kinetics of antibody appearance using these assays in 22 patients after they were tested positive by RT-PCR. We observed great heterogeneity in antibody detection post-symptom onset. The median antibody detection time was between 8 and 10 days according to the manufacturers. All the tests showed a sensitivity of 60 to 80% on day 10 and 100% on day 15. In addition, a single cross-reaction was observed with other human coronavirus infections. Thus, immunochromatographic tests for the detection of anti-SARS-CoV-2 antibodies may have their place for the diagnostic panel of COVID-19.
Keywords: Antibody; COVID-19; Lateral flow assay; SARS-CoV-2.
Copyright © 2020 The British Infection Association. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest All authors have no conflict of interest to declare
Figures

Comment in
-
Elevated nucleoprotein-induced interferon-γ release in COVID-19 patients detected in a SARS-CoV-2 enzyme-linked immunosorbent spot assay.J Infect. 2020 Sep;81(3):452-482. doi: 10.1016/j.jinf.2020.06.015. Epub 2020 Jun 12. J Infect. 2020. PMID: 32540458 Free PMC article. No abstract available.
References
-
- Guo L., Ren L., Yang S., Xiao M., Chang D., Yang F., Dela Cruz C.S., Wang Y., Wu C., Xiao Y., Zhang L., Han L., Dang S., Xu Yan, Yang Q., Xu S., Zhu H., Xu Yingchun, Jin Q., Sharma L., Wang L., Wang J. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19) Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020 doi: 10.1093/cid/ciaa310. - DOI - PMC - PubMed
-
- Haveri A., Smura T., Kuivanen S., Österlund P., Hepojoki J., Ikonen N., Pitkäpaasi M., Blomqvist S., Rönkkö E., Kantele A., Strandin T., Kallio-Kokko H., Mannonen L., Lappalainen M., Broas M., Jiang M., Siira L., Salminen M., Puumalainen T., Sane J., Melin M., Vapalahti O., Savolainen-Kopra C. Serological and molecular findings during SARS-CoV-2 infection: the first case study in Finland, January to February 2020. Euro Surveill. Bull. Eur. Sur Mal. Transm. Eur. Commun. Dis. Bull. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.11.2000266. - DOI - PMC - PubMed
-
- Li Z., Yi Y., Luo X., Xiong N., Liu Y., Li S., Sun R., Wang Y., Hu B., Chen W., Zhang Y., Wang J., Huang B., Lin Y., Yang J., Cai W., Wang X., Cheng J., Chen Z., Sun K., Pan W., Zhan Z., Chen L., Ye F. Development and Clinical Application of A Rapid IgM-IgG Combined Antibody Test for SARS-CoV-2 Infection Diagnosis. J. Med. Virol. 2020 doi: 10.1002/jmv.25727. - DOI - PMC - PubMed
-
- Linton N.M., Kobayashi T., Yang Y., Hayashi K., Akhmetzhanov A.R., Jung S.-M., Yuan B., Kinoshita R., Nishiura H. Incubation Period and Other Epidemiological Characteristics of 2019 Novel Coronavirus Infections with Right Truncation: A Statistical Analysis of Publicly Available Case Data. J. Clin. Med. 2020;9 doi: 10.3390/jcm9020538. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

