A multicompartment vascular implant of electrospun wintergreen oil/ polycaprolactone fibers coated with poly(ethylene oxide)
- PMID: 32389823
- PMCID: PMC8640569
- DOI: 10.1016/j.bj.2020.04.008
A multicompartment vascular implant of electrospun wintergreen oil/ polycaprolactone fibers coated with poly(ethylene oxide)
Abstract
Background: The aim of the present study was to fabricate double layered scaffolds of electrospun polycaprolactone (PCL) and poly(ethylene oxide) (PEO). The electrospun PCL fibers were functionalized with wintergreen oil (WO) as a novel approach to prevent vascular grafts failure due to thrombosis by adjusting biomaterial-blood interactions.
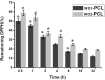
Methods: PCL tubular scaffolds were prepared by electrospinning approach and coated with PEO as a hydrophilic polymer. The single and double layered scaffolds were characterized in terms of their morphological, chemical properties -as well as-hemocompatibility assays (i.e. prothrombin time, hemolysis percentage and platelets adhesion). Moreover, the antioxidant potential of WO-PCL samples were measured by 2,2-diphenyl-1-picrylhydrazyl hydrate (DPPH) free radical assay.
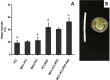
Results: The results demonstrated that incorporation of WO during the electrospinning process decreased the PCL fiber diameter. In addition, the prothrombine time assay shows that WO could be used to lower the electrospun PCL fiber tendency to induce blood clotting. Moreover, SEM observations of platelets adhesion of both single and double layered PCL/PEO scaffolds fiber shows an increase of platelets number, compared with the scaffolds containing WO.
Conclusions: The antioxidant potential and blood compatibility measurements of WO-PCL/PEO samples highlight the approach made so far as an ideal synthetic small size vascular grafts to overcome autogenous grafts shortages and drawbacks.
Keywords: Blood compatibility; Electrospun vascular grafts; Poly(ethylene oxide) (PEO); Polycaprolactone (PCL); Wintergreen oil (WO).
Copyright © 2020 Chang Gung University. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflicts of Interest The authors have no conflicts of interest to disclose.
Figures





References
-
- Acar C., Ramsheyi A., Pagny J.Y., Jebara V., Barrier P., Fabiani J.N., et al. The radial artery for coronary artery bypass grafting: clinical and angiographic results at five years. J Thorac Cardiovasc Surg. 1998;116:981–989. - PubMed
-
- Spray T.L., Roberts W.C. Changes in saphenous veins used as aortocoronary bypass grafts. Am Heart J. 1977;94:500–516. - PubMed
-
- Cox J.L., Chiasson D.A., Gotlieb A.I. Stranger in a strange land: the pathogenesis of saphenous vein graft stenosis with emphasis on structural and functional differences between veins and arteries. Prog Cardiovasc Dis. 1991;34:45–68. - PubMed
-
- Matsumura G., Hibino N., Ikada Y., Kurosawa H., Shin'oka T. Successful application of tissue engineered vascular autografts: clinical experience. Biomaterials. 2003;24:2303–2308. - PubMed
-
- Hoenig M.R., Campbell G.R., Rolfe B.E., Campbell J.H. Tissue-engineered blood vessels: alternative to autologous grafts? Arterioscler Thromb Vasc Biol. 2005;25:1128–1134. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

