Surface analysis tools for characterizing biological materials
- PMID: 32390029
- PMCID: PMC7337324
- DOI: 10.1039/d0cs00181c
Surface analysis tools for characterizing biological materials
Abstract
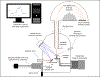
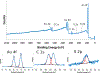
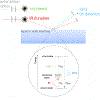
Surfaces represent a unique state of matter that typically have significantly different compositions and structures from the bulk of a material. Since surfaces are the interface between a material and its environment, they play an important role in how a material interacts with its environment. Thus, it is essential to characterize, in as much detail as possible, the surface structure and composition of a material. However, this can be challenging since the surface region typically is only minute portion of the entire material, requiring specialized techniques to selectively probe the surface region. This tutorial will provide a brief review of several techniques used to characterize the surface and interface regions of biological materials. For each technique we provide a description of the key underlying physics and chemistry principles, the information provided, strengths and weaknesses, the types of samples that can be analyzed, and an example application. Given the surface analysis challenges for biological materials, typically there is never just one technique that can provide a complete surface characterization. Thus, a multi-technique approach to biological surface analysis is always required.
Conflict of interest statement
Conflicts of interest
There are no conflicts of interest to declare.
Figures










References
-
- Vickerman JC and Gilmore IS, Surface Analysis: The Principal Techniques, John Wiley & Sons, Chichester, UK, 2009.
-
- Castner DG and Ratner BD, Surface Science, 2002, 500, 28–60.
-
- Ratner BD, Weathersby PK, Hoffman AS, Kelly MA and Scharpen LH, Journal of Applied Polymer Science, 1978, 22, 643–664.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

