Tau immunophenotypes in chronic traumatic encephalopathy recapitulate those of ageing and Alzheimer's disease
- PMID: 32390044
- PMCID: PMC7241956
- DOI: 10.1093/brain/awaa071
Tau immunophenotypes in chronic traumatic encephalopathy recapitulate those of ageing and Alzheimer's disease
Abstract
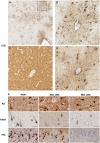
Traumatic brain injury (TBI) is a risk factor for neurodegenerative disease, including chronic traumatic encephalopathy (CTE). Preliminary consensus criteria define the pathognomonic lesion of CTE as patchy tau pathology within neurons and astrocytes at the depths of cortical sulci. However, the specific tau isoform composition and post-translational modifications in CTE remain largely unexplored. Using immunohistochemistry, we performed tau phenotyping of CTE neuropathologies and compared this to a range of tau pathologies, including Alzheimer's disease, primary age-related tauopathy, ageing-related tau astrogliopathy and multiple subtypes of frontotemporal lobar degeneration with tau inclusions. Cases satisfying preliminary consensus diagnostic criteria for CTE neuropathological change (CTE-NC) were identified (athletes, n = 10; long-term survivors of moderate or severe TBI, n = 4) from the Glasgow TBI Archive and Penn Neurodegenerative Disease Brain Bank. In addition, material from a range of autopsy-proven ageing-associated and primary tauopathies in which there was no known history of exposure to TBI was selected as non-injured controls (n = 32). Each case was then stained with a panel of tau antibodies specific for phospho-epitopes (PHF1, CP13, AT100, pS262), microtubule-binding repeat domains (3R, 4R), truncation (Tau-C3) or conformation (GT-7, GT-38) and the extent and distribution of staining assessed. Cell types were confirmed with double immunofluorescent labelling. Results demonstrate that astroglial tau pathology in CTE is composed of 4R-immunoreactive thorn-shaped astrocytes, echoing the morphology and immunophenotype of astrocytes encountered in ageing-related tau astrogliopathy. In contrast, neurofibrillary tangles of CTE contain both 3R and 4R tau, with post-translational modifications and conformations consistent with Alzheimer's disease and primary age-related tauopathy. Our observations establish that the astroglial and neurofibrillary tau pathologies of CTE are phenotypically distinct from each other and recapitulate the tau immunophenotypes encountered in ageing and Alzheimer's disease. As such, the immunohistochemical distinction of CTE neuropathology from other mixed 3R/4R tauopathies of Alzheimer's disease and ageing may rest solely on the pattern and distribution of pathology.
Keywords: TBI; ageing-related tau astrogliopathy; chronic traumatic encephalopathy; tau; traumatic brain injury.
© The Author(s) (2020). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures




References
-
- Abraha A, Ghoshal N, Gamblin TC, Cryns V, Berry RW, Kuret J, et al.C-terminal inhibition of tau assembly in vitro and in Alzheimer’s disease. J Cell Sci 2000; 113 (Pt 21): 3737–45. - PubMed
-
- Arai T, Ikeda K, Akiyama H, Shikamoto Y, Tsuchiya K, Yagishita S, et al.Distinct isoforms of tau aggregated in neurons and glial cells in brains of patients with Pick’s disease, corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol 2001; 101: 167–73. - PubMed
-
- Berry RW, Abraha A, Lagalwar S, LaPointe N, Gamblin TC, Cryns VL, et al.Inhibition of tau polymerization by its carboxy-terminal caspase cleavage fragment. Biochemistry 2003; 42: 8325–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AG010124/AG/NIA NIH HHS/United States
- RF1 AG054991/AG/NIA NIH HHS/United States
- R01 NS038104/NS/NINDS NIH HHS/United States
- R01 NS094003/NS/NINDS NIH HHS/United States
- P01 AG066597/AG/NIA NIH HHS/United States
- F32 AG053036/AG/NIA NIH HHS/United States
- U54 NS115322/NS/NINDS NIH HHS/United States
- TL1 TR001880/TR/NCATS NIH HHS/United States
- R01 NS092398/NS/NINDS NIH HHS/United States
- G0701018/MRC_/Medical Research Council/United Kingdom
- G1100578/MRC_/Medical Research Council/United Kingdom
- P01 AG009215/AG/NIA NIH HHS/United States
- MR/N004272/1/MRC_/Medical Research Council/United Kingdom
- P01 AG017586/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

