Baseline clinical characteristics and disease burden in patients with paroxysmal nocturnal hemoglobinuria (PNH): updated analysis from the International PNH Registry
- PMID: 32390114
- PMCID: PMC7316848
- DOI: 10.1007/s00277-020-04052-z
Baseline clinical characteristics and disease burden in patients with paroxysmal nocturnal hemoglobinuria (PNH): updated analysis from the International PNH Registry
Abstract
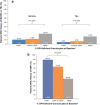
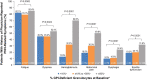
The International Paroxysmal Nocturnal Hemoglobinuria (PNH) Registry (NCT01374360) was initiated to optimize patient management by collecting data regarding disease burden, progression, and clinical outcomes. Herein, we report updated baseline demographics, clinical characteristics, disease burden data, and observed trends regarding clone size in the largest cohort of Registry patients. Patients with available data as of July 2017 were stratified by glycosylphosphatidylinositol (GPI)-deficient granulocyte clone size (< 10%, ≥ 10%-< 50%, and ≥ 50%). All patients were untreated with eculizumab at baseline, defined as date of eculizumab initiation or date of Registry enrollment (if never treated with eculizumab). Outcomes assessed in the current analysis included proportions of patients with high disease activity (HDA), history of major adverse vascular events (MAVEs; including thrombotic events [TEs]), bone marrow failure (BMF), red blood cell (RBC) transfusions, and PNH-related symptoms. A total of 4439 patients were included, of whom 2701 (60.8%) had available GPI-deficient granulocyte clone size data. Among these, median clone size was 31.8% (1002 had < 10%; 526 had ≥ 10%-< 50%; 1173 had ≥ 50%). There were high proportions of patients with HDA (51.6%), history of MAVEs (18.8%), BMF (62.6%), RBC transfusion (61.3%), and impaired renal function (42.8%). All measures except RBC transfusion history significantly correlated with GPI-deficient granulocyte clone size. A large proportion of patients with GPI-deficient granulocyte clone size < 10% had hemolysis (9.7%), MAVEs (10.2%), HDA (9.1%), and/or PNH-related symptoms. Although larger GPI-deficient granulocyte clone sizes were associated with higher disease burden, a substantial proportion of patients with smaller clone sizes had history of MAVEs/TEs.
Keywords: Bone marrow failure; Eculizumab; Health-related quality of life; Paroxysmal nocturnal hemoglobinuria; Registries; Thrombosis.
Conflict of interest statement
Hubert Schrezenmeier has received honoraria and research support (both to University of Ulm) from Alexion Pharmaceuticals, Inc., and honoraria (to University of Ulm) from Ra Pharma, Roche, and Alnylam Pharmaceuticals. Alexander Röth has received honoraria and consulting fees from Alexion Pharmaceuticals, Inc., Roche, and Novartis, and research support from Alexion Pharmaceuticals, Inc., and Roche. David J. Araten has received honoraria and consulting fees from Alexion Pharmaceuticals, Inc. Yuzuru Kanakura has received honoraria and research support from Alexion Pharmaceuticals, Inc. Loree Larratt has received honoraria and research support (for the laboratory at the University of Alberta, for ADAMTS13 testing) from Alexion Pharmaceuticals, Inc. Jamile M. Shammo has received honoraria, consulting fees, and research support from Alexion Pharmaceuticals, Inc. Amanda Wilson and Gilda Shayan are former employees and stockholders of Alexion Pharmaceuticals, Inc. Jaroslaw P. Maciejewski has received consulting fees from Alexion Pharmaceuticals, Inc., Apellis Pharmaceuticals, and Ra Pharma; has also received speaker fees from Alexion Pharmaceuticals, Inc. and is a member of the Executive Committee of International PNH Registry for Alexion Pharmaceuticals, Inc.
Figures

References
-
- Jang JH, Kim JS, Yoon SS, Lee JH, Kim YK, Jo DY, Chung J, Sohn SK, Lee JW. Predictive factors of mortality in population of patients with paroxysmal nocturnal hemoglobinuria (PNH): results from a Korean PNH registry. J Korean Med Sci. 2016;31(2):214–221. doi: 10.3346/jkms.2016.31.2.214. - DOI - PMC - PubMed
-
- Parker CJ (2011) Management of paroxysmal nocturnal hemoglobinuria in the era of complement inhibitory therapy. Hematology Am Soc Hematol Educ Program 2011:21-29 10.1182/asheducation-2011.1.21 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

