Vitamin D and the hepatitis B vaccine response: a prospective cohort study and a randomized, placebo-controlled oral vitamin D3 and simulated sunlight supplementation trial in healthy adults
- PMID: 32390123
- PMCID: PMC7867563
- DOI: 10.1007/s00394-020-02261-w
Vitamin D and the hepatitis B vaccine response: a prospective cohort study and a randomized, placebo-controlled oral vitamin D3 and simulated sunlight supplementation trial in healthy adults
Abstract
Purpose: To determine serum 25(OH)D and 1,25(OH)2D relationship with hepatitis B vaccination (study 1). Then, to investigate the effects on hepatitis B vaccination of achieving vitamin D sufficiency (serum 25(OH)D ≥ 50 nmol/L) by a unique comparison of simulated sunlight and oral vitamin D3 supplementation in wintertime (study 2).
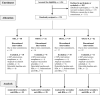
Methods: Study 1 involved 447 adults. In study 2, 3 days after the initial hepatitis B vaccination, 119 men received either placebo, simulated sunlight (1.3 × standard-erythema dose, 3 × /week for 4 weeks and then 1 × /week for 8 weeks) or oral vitamin D3 (1000 IU/day for 4 weeks and 400 IU/day for 8 weeks). We measured hepatitis B vaccination efficacy as percentage of responders with anti-hepatitis B surface antigen immunoglobulin G ≥ 10 mIU/mL.
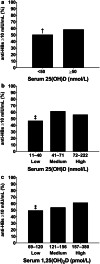
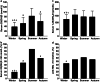
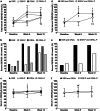
Results: In study 1, vaccine response was poorer in persons with low vitamin D status (25(OH)D ≤ 40 vs 41-71 nmol/L mean difference [95% confidence interval] - 15% [- 26, - 3%]; 1,25(OH)2D ≤ 120 vs ≥ 157 pmol/L - 12% [- 24%, - 1%]). Vaccine response was also poorer in winter than summer (- 18% [- 31%, - 3%]), when serum 25(OH)D and 1,25(OH)2D were at seasonal nadirs, and 81% of persons had serum 25(OH)D < 50 nmol/L. In study 2, vitamin D supplementation strategies were similarly effective in achieving vitamin D sufficiency from the winter vitamin D nadir in almost all (~ 95%); however, the supplementation beginning 3 days after the initial vaccination did not effect the vaccine response (vitamin D vs placebo 4% [- 21%, 14%]).
Conclusion: Low vitamin D status at initial vaccination was associated with poorer hepatitis B vaccine response (study 1); however, vitamin D supplementation commencing 3 days after vaccination (study 2) did not influence the vaccination response.
Clinical trial registry number: Study 1 NCT02416895; https://clinicaltrials.gov/ct2/show/study/NCT02416895 ; Study 2 NCT03132103; https://clinicaltrials.gov/ct2/show/NCT03132103 .
Keywords: 25-Hydroxyvitamin D; Cholecalciferol; Hepatitis B; UVB; Vaccination; Vitamin D.
Conflict of interest statement
None of the authors report a conflict of interest related to the study.
Figures
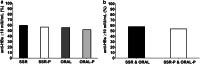
References
-
- He CS, Handzlik M, Fraser WD, Muhamad A, Preston H, Richardson A, Gleeson M. Influence of vitamin D status on respiratory infection incidence and immune function during 4 months of winter training in endurance sport athletes. Exerc Immunol Rev. 2013;19:86–101. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

