Lipopolysaccharides transport during fat absorption in rodent small intestine
- PMID: 32390462
- PMCID: PMC7311662
- DOI: 10.1152/ajpgi.00079.2020
Lipopolysaccharides transport during fat absorption in rodent small intestine
Abstract
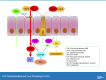
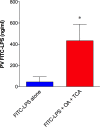
Lipopolysaccharides (LPS) are potent pro-inflammatory molecules that enter the systemic circulation from the intestinal lumen by uncertain mechanisms. We investigated these mechanisms and the effect of exogenous glucagon-like peptide-2 (GLP-2) on LPS transport in the rodent small intestine. Transmucosal LPS transport was measured in Ussing-chambered rat jejunal mucosa. In anesthetized rats, the appearance of fluorescein isothiocyanate (FITC)-LPS into the portal vein (PV) and the mesenteric lymph was simultaneously monitored after intraduodenal perfusion of FITC-LPS with oleic acid and taurocholate (OA/TCA). In vitro, luminally applied LPS rapidly appeared in the serosal solution only with luminal OA/TCA present, inhibited by the lipid raft inhibitor methyl-β-cyclodextrin (MβCD) and the CD36 inhibitor sulfosuccinimidyl oleate (SSO), or by serosal GLP-2. In vivo, perfusion of FITC-LPS with OA/TCA rapidly increased FITC-LPS appearance into the PV, followed by a gradual increase of FITC-LPS into the lymph. Rapid PV transport was inhibited by the addition of MβCD or by SSO, whereas transport into the lymph was inhibited by chylomicron synthesis inhibition. Intraveous injection of the stable GLP-2 analog teduglutide acutely inhibited FITC-LPS transport into the PV, yet accelerated FITC-LPS transport into the lymph via Nω-nitro-l-arginine methyl ester (l-NAME)- and PG97-269-sensitive mechanisms. In vivo confocal microscopy in mouse jejunum confirmed intracellular FITC-LPS uptake with no evidence of paracellular localization. This is the first direct demonstration in vivo that luminal LPS may cross the small intestinal barrier physiologically during fat absorption via lipid raft- and CD36-mediated mechanisms, followed by predominant transport into the PV, and that teduglutide inhibits LPS uptake into the PV in vivo.NEW & NOTEWORTHY We report direct in vivo confirmation of transcellular lipopolysaccharides (LPS) uptake from the intestine into the portal vein (PV) involving CD36 and lipid rafts, with minor uptake via the canonical chylomicron pathway. The gut hormone glucagon-like peptide-2 (GLP-2) inhibited uptake into the PV. These data suggest that the bulk of LPS absorption is via the PV to the liver, helping clarify the mechanism of LPS transport into the PV as part of the "gut-liver" axis. These data do not support the paracellular transport of LPS, which has been implicated in the pathogenesis of the "leaky gut" syndrome.
Keywords: CD36; GLP-2; LCFA absorption; LPS transport; lipid raft.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

