Mechanosensing of Mechanical Confinement by Mesenchymal-Like Cells
- PMID: 32390868
- PMCID: PMC7193100
- DOI: 10.3389/fphys.2020.00365
Mechanosensing of Mechanical Confinement by Mesenchymal-Like Cells
Abstract
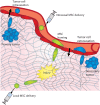
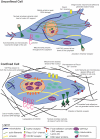
Mesenchymal stem cells (MSCs) and tumor cells have the unique capability to migrate out of their native environment and either home or metastasize, respectively, through extremely heterogeneous environments to a distant location. Once there, they can either aid in tissue regrowth or impart an immunomodulatory effect in the case of MSCs, or form secondary tumors in the case of tumor cells. During these journeys, cells experience physically confining forces that impinge on the cell body and the nucleus, ultimately causing a multitude of cellular changes. Most drastically, confining individual MSCs within hydrogels or confining monolayers of MSCs within agarose wells can sway MSC lineage commitment, while applying a confining compressive stress to metastatic tumor cells can increase their invasiveness. In this review, we seek to understand the signaling cascades that occur as cells sense confining forces and how that translates to behavioral changes, including elongated and multinucleated cell morphologies, novel migrational mechanisms, and altered gene expression, leading to a unique MSC secretome that could hold great promise for anti-inflammatory treatments. Through comparison of these altered behaviors, we aim to discern how MSCs alter their lineage selection, while tumor cells may become more aggressive and invasive. Synthesizing this information can be useful for employing MSCs for therapeutic approaches through systemic injections or tissue engineered grafts, and developing improved strategies for metastatic cancer therapies.
Keywords: cancer; confinement; differentiation; migration; stem cell.
Copyright © 2020 Doolin, Moriarty and Stroka.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

