The Current and Future State of Vaccines, Antivirals and Gene Therapies Against Emerging Coronaviruses
- PMID: 32390971
- PMCID: PMC7193113
- DOI: 10.3389/fmicb.2020.00658
The Current and Future State of Vaccines, Antivirals and Gene Therapies Against Emerging Coronaviruses
Abstract
Emerging coronaviruses (CoV) are constant global public health threats to society. Multiple ongoing clinical trials for vaccines and antivirals against CoVs showcase the availability of medical interventions to both prevent and treat the future emergence of highly pathogenic CoVs in human. However, given the diverse nature of CoVs and our close interactions with wild, domestic and companion animals, the next epidemic zoonotic CoV could resist the existing vaccines and antivirals developed, which are primarily focused on Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) and Middle East Respiratory Syndrome Coronavirus (MERS CoV). In late 2019, the novel CoV (SARS-CoV-2) emerged in Wuhan, China, causing global public health concern. In this review, we will summarize the key advancements of current vaccines and antivirals against SARS-CoV and MERS-CoV as well as discuss the challenge and opportunity in the current SARS-CoV-2 crisis. At the end, we advocate the development of a "plug-and-play" platform technologies that could allow quick manufacturing and administration of broad-spectrum countermeasures in an outbreak setting. We will discuss the potential of AAV-based gene therapy technology for in vivo therapeutic antibody delivery to combat SARS-CoV-2 outbreak and the future emergence of severe CoVs.
Keywords: 2019 nCoV; MERS- and SARS-CoV; adeno-associate virus; antivirals; coronavirus (CoV); passive immunization strategy; vaccine.
Copyright © 2020 Tse, Meganck, Graham and Baric.
Figures
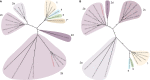
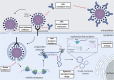
References
-
- Agnihothram S., Gopal R., Yount B. L. J., Donaldson E. F., Menachery V. D., Graham R. L., et al. (2014). Evaluation of serologic and antigenic relationships between middle eastern respiratory syndrome coronavirus and other coronaviruses to develop vaccine platforms for the rapid response to emerging coronaviruses. J. Infect. Dis. 209 995–1006. 10.1093/infdis/jit609 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

