Necrostatin-1 Ameliorates Neutrophilic Inflammation in Asthma by Suppressing MLKL Phosphorylation to Inhibiting NETs Release
- PMID: 32391007
- PMCID: PMC7194114
- DOI: 10.3389/fimmu.2020.00666
Necrostatin-1 Ameliorates Neutrophilic Inflammation in Asthma by Suppressing MLKL Phosphorylation to Inhibiting NETs Release
Abstract
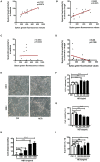
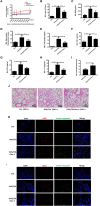
Neutrophilic inflammation occurs during asthma exacerbation, and especially, in patients with steroid-refractory asthma, but the underlying mechanisms are poorly understood. Recently, a significant accumulation of neutrophil extracellular traps (NETs) in the airways of neutrophilic asthma has been documented, suggesting that NETs may play an important role in the pathogenesis. In this study, we firstly demonstrated that NETs could induce human airway epithelial cell damage in vitro. In a mouse asthmatic model of neutrophil-dominated airway inflammation, we found that NETs were markedly increased in bronchoalveolar lavage (BAL), and the formation of NETs exacerbated the airway inflammation. Additionally, a small-molecule drug necrostatin-1 (Nec-1) shown to inhibit NETs formation was found to alleviate the neutrophil-dominated airway inflammation. Nec-1 reduced total protein concentration, myeloperoxidase activity, and the levels of inflammatory cytokines in BAL. Finally, further experiments proved that the inhibition of Nec-1 on NETs formation might be related to its ability to inhibiting mixed lineage kinase domain-like (MLKL) phosphorylation and perforation. Together, these results document that NETs are closely associated with the pathogenesis of neutrophilic asthma and inhibition of the formation of NETs by Nec-1 may be a new therapeutic strategy to ameliorate neutrophil-dominated airway inflammation.
Keywords: MLKL; Necrostatin-1; asthma; neutrophil extracellular traps; neutrophilic inflammation.
Copyright © 2020 Han, Jie, Wang, Zhang, Wang, Yu, Zhang, He, Chen, Lai and Sun.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

