Serological Approaches for COVID-19: Epidemiologic Perspective on Surveillance and Control
- PMID: 32391022
- PMCID: PMC7194125
- DOI: 10.3389/fimmu.2020.00879
Serological Approaches for COVID-19: Epidemiologic Perspective on Surveillance and Control
Abstract
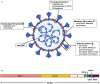
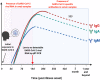
Since December 2019, the novel coronavirus, SARS-CoV-2, has garnered global attention due to its rapid transmission, which has infected more than two million people worldwide. Early detection of SARS-CoV-2 is one of the crucial interventions to control virus spread and dissemination. Molecular assays have been the gold standard to directly detect for the presence of viral genetic material in infected individuals. However, insufficient viral RNA at the point of detection may lead to false negative results. As such, it is important to also employ immune-based assays to determine one's exposure to SARS-CoV-2, as well as to assist in the surveillance of individuals with prior exposure to SARS-CoV-2. Within a span of 4 months, extensive studies have been done to develop serological systems to characterize the antibody profiles, as well as to identify and generate potentially neutralizing antibodies during SARS-CoV-2 infection. The vast diversity of novel findings has added value to coronavirus research, and a strategic consolidation is crucial to encompass the latest advances and developments. This review aims to provide a concise yet extensive collation of current immunoassays for SARS-CoV-2, while discussing the strengths, limitations and applications of antibody detection in SARS-CoV-2 research and control.
Keywords: COVID-19; SARS-CoV-2; antibodies; detection; immunoassays; nucleocapsid; receptor binding domain; spike.
Copyright © 2020 Lee, Lin, Renia and Ng.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

