Upregulated MELK Leads to Doxorubicin Chemoresistance and M2 Macrophage Polarization via the miR-34a/JAK2/STAT3 Pathway in Uterine Leiomyosarcoma
- PMID: 32391256
- PMCID: PMC7188922
- DOI: 10.3389/fonc.2020.00453
Upregulated MELK Leads to Doxorubicin Chemoresistance and M2 Macrophage Polarization via the miR-34a/JAK2/STAT3 Pathway in Uterine Leiomyosarcoma
Abstract
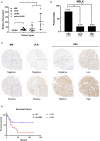
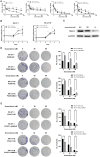
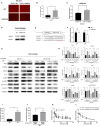
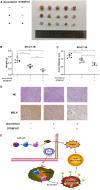
Uterine leiomyosarcoma (ULMS) is the most lethal gynecologic malignancy with few therapeutic options. Chemoresistance prevails as a major hurdle in treating this malignancy, yet the mechanism of chemoresistance remains largely unclear. In this study, we certified MELK as a poor prognostic marker through bioinformatic analysis of the GEO database. Cellular experiments in vitro revealed that MELK played an essential role in ULMS cells' chemoresistance and that a high expression of MELK could lead to doxorubicin resistance. mRNA profiling uncovered the pathways that MELK was involved in which led to doxorubicin resistance. MELK was found to affect ULMS cells' chemoresistance through an anti-apoptotic mechanism via the JAK2/STAT3 pathway. miRNA profiling also revealed that upregulated MELK could induce the decrease of miRNA-34a (regulated by JAK2/STAT3 pathway). We detected that MELK overexpression could induce M2 macrophage polarization via the miR-34a/JAK2/STAT3 pathway, contributing to doxorubicin chemoresistance in the tumor microenvironment. OTSSP167, a MELK inhibitor, may increase ULMS sensitivity to doxorubicin. Our investigation could propose novel targets for early diagnosis and precision therapy in ULMS patients.
Keywords: JAK2; M2 macrophage; MELK; STAT3; apoptosis; chemoresistance; uterine leiomyosarcoma.
Copyright © 2020 Zhang, Sun, Li, Jiao, Griffin, Dongol, Wu, Zhang, Cao, Dong, Yang, Zhang and Kong.
Figures

References
-
- Tominaga T, Abe O, Enomoto K, Abe R, Iino Y, Koyama H, et al. A randomized controlled study of (2"R)-4'-O-tetrahydropyranyladriamycin and adriamycin in combination with cyclophosphamide and 5-fluorouracil in the treatment of advanced and recurrent breast cancer. Clinical Study Group of THP for breast cancer in Japan. Biomed Pharmacother. (1989) 43:271–8. 10.1016/0753-3322(89)90008-5 - DOI - PubMed
-
- Pautier P, Floquet A, Penel N, Piperno-Neumann S, Isambert N, Rey A, et al. Randomized multicenter and stratified phase II study of gemcitabine alone versus gemcitabine and docetaxel in patients with metastatic or relapsed leiomyosarcomas: a Federation Nationale des Centres de Lutte Contre le Cancer (FNCLCC) French Sarcoma Group Study (TAXOGEM study). Oncologist. (2012) 17:1213–20. 10.1634/theoncologist.2011-0467 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

