New Screening Test Improves Detection of Prostate Cancer Using Circulating Tumor Cells and Prostate-Specific Markers
- PMID: 32391268
- PMCID: PMC7192049
- DOI: 10.3389/fonc.2020.00582
New Screening Test Improves Detection of Prostate Cancer Using Circulating Tumor Cells and Prostate-Specific Markers
Abstract
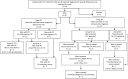
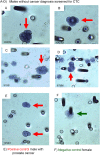
The current screening-test for prostate cancer, affecting 10% of men worldwide, has a high false negative rate and a low true positive rate. A more reliable screening test is needed. Circulating-Tumor-Cells (CTC) provide a biomarker for early carcinogenesis, cancer progression and treatment effectiveness. The cytology-based ISET®-CTC Test is a clinically validated blood test with high sensitivity and specificity. This study aimed to evaluate the ISET®-CTC test combined with prostate-specific-marker staining as a screening test for the detection of prostate cancer. We selected a group of 47 men from our ongoing CTC screening study involving 2,000 patient-tests from Sep-2014 to July-2019, who also underwent standard diagnostic cancer testing before or after CTC testing. While 20 of the 47 men were diagnosed with prostate cancer before the ISET®-CTC test, 27 men underwent screening. We studied the CTC identified in 45 CTC-positive men by Immuno-Cyto-Chemistry (ICC) assays with the prostate-specific-marker PSA. CTC were ICC-PSA-marker positive in all men diagnosed with primary prostate cancer (n = 20). Secondary cancers were detected in 63% (n = 7/11) of men with mixed CTC-population (ICC-PSA-positive/ICC-PSA-negative). Of the 27 men screened, 25 had CTC, and 84% of those (n = 20) were positive for the prostate-specific-PSA-marker. Follow-up testing suggested suspected prostate cancer in 20/20 men by a positive PSMA-PET scan, and biopsies performed in 45% (n = 9/20) men confirmed the diagnosis of early prostate cancer. Kidney cancer or B-cell lymphoma were detected in two men with ICC-PSA-marker negative CTC. Our study suggests that the combination of ISET®-CTC and ICC-PSA-marker-testing has an estimated positive-predictive-value (PPV) of 99% and a negative-predictive-value (NPV) of 97%, providing a more reliable screening test for prostate cancer than the standard PSA-blood-test (PPV = 25%; NPV = 15.5%). Our findings warrant further studies to evaluate the new test's potential for prostate cancer screening on a population level.
Keywords: cancer screening; circulating tumor cells (CTC); early detection; prostate cancer; prostate specific antigen (PSA).
Copyright © 2020 Ried, Tamanna, Matthews, Eng and Sali.
Figures
References
-
- Cancer Australia. Prostate Cancer Statistics. (2019). Available online at: https://prostate-cancer.canceraustralia.gov.au/statistics (accessed March 15, 2020).
-
- National Cancer Institute USA Prostate-Specific-Antigen (PSA) Test Fact Sheet. (2017). Available online at: www.cancer.gov/types/prostate/psa-fact-sheet (accessed March 15, 2020).
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

