Promise and Progress of an HIV-1 Cure by Adeno-Associated Virus Vector Delivery of Anti-HIV-1 Biologics
- PMID: 32391289
- PMCID: PMC7190809
- DOI: 10.3389/fcimb.2020.00176
Promise and Progress of an HIV-1 Cure by Adeno-Associated Virus Vector Delivery of Anti-HIV-1 Biologics
Abstract
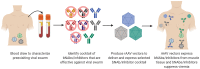
Despite the success of antiretroviral therapy (ART) at suppressing HIV-1 infection, a cure that eradicates all HIV-1-infected cells has been elusive. The latent viral reservoir remains intact in tissue compartments that are not readily targeted by the host immune response that could accelerate the rate of reservoir decline during ART. However, over the past decade, numerous broadly neutralizing antibodies (bNAbs) have been discovered and characterized. These bNAbs have also given rise to engineered antibody-like inhibitors that are just as or more potent than bNAbs themselves. The question remains whether bNAbs and HIV-1 inhibitors will be the effective "kill" to a shock-and-kill approach to eliminate the viral reservoir. Additional research over the past few years has sought to develop recombinant adeno-associated virus (rAAV) vectors to circumvent the need for continual administration of bNAbs and maintain persistent expression in a host. This review discusses the advancements made in using rAAV vectors for the delivery of HIV-1 bNAbs and inhibitors and the future of this technology in HIV-1 cure research. Numerous groups have demonstrated with great efficacy that rAAV vectors can successfully express protective concentrations of bNAbs and HIV-1 inhibitors. Yet, therapeutic concentrations, especially in non-human primate (NHP) models, are not routinely achieved. As new studies have been reported, more challenges have been identified for utilizing rAAV vectors, specifically how the host immune response limits the attainable concentrations of bNAbs and inhibitors. The next few years should provide improvements to rAAV vector delivery that will ultimately show whether they can be used for expressing bNAbs and HIV-1 inhibitors to eliminate the HIV-1 viral reservoir.
Keywords: HIV-1; HIV-1 cure; adeno-associate virus; broadly neutralizing antibodies (bNAbs); eCD4-Ig.
Copyright © 2020 Gardner.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

