Sequence Changes Modulate Peptoid Self-Association in Water
- PMID: 32391314
- PMCID: PMC7191062
- DOI: 10.3389/fchem.2020.00260
Sequence Changes Modulate Peptoid Self-Association in Water
Abstract
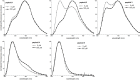
Peptoids, N-substituted glycine oligomers, are a class of diverse and sequence-specific peptidomimetics with wide-ranging applications. Advancing the functional repertoire of peptoids to emulate native peptide and protein functions requires engineering peptoids that adopt regular secondary and tertiary structures. An understanding of how changes to peptoid sequence change structural features, particularly in water-soluble systems, is underdeveloped. To address this knowledge gap, five 15-residue water-soluble peptoids that include naphthalene-functionalized side chains were designed, prepared, and subjected to a structural study using a palette of techniques. Peptoid sequence designs were based on a putative amphiphilic helix peptoid bearing structure-promoting (S)-N-(1-naphthylethyl)glycine residues whose self-association in water has been studied previously. New peptoid variants reported here include sequence changes that influenced peptoid conformational flexibility, functional group patterning (amphiphilicity), and hydrophobicity. Peptoid structures were evaluated and compared using circular dichroism spectroscopy, fluorescence spectroscopy, and size exclusion chromatography. Spectral data confirmed that sequence changes alter peptoids' degree of assembly and the organization of self-assembled structures in aqueous solutions. Insights gained in these studies will inform the design of new water-soluble peptoids with regular structural features, including desirable higher-order (tertiary and quaternary) structural features.
Keywords: circular dichroism (CD) spectroscopy; fluorescence spectroscopy; peptidomimetic; peptoid; self-association; size exclusion chromatography.
Copyright © 2020 Fuller, Jimenez, Martinetto, Moreno, Calkins, Dowell, Huber, McComas and Ortega.
Figures






Similar articles
-
Solution effects on the self-association of a water-soluble peptoid.Biopolymers. 2019 Apr;110(4):e23248. doi: 10.1002/bip.23248. Epub 2018 Dec 22. Biopolymers. 2019. PMID: 30578630
-
Use of the environmentally sensitive fluorophore 4-N,N-dimethylamino-1,8-naphthalimide to study peptoid helix structures.Biopolymers. 2011;96(5):627-38. doi: 10.1002/bip.21605. Biopolymers. 2011. PMID: 22180910
-
Self-association of water-soluble peptoids comprising (S)-N-1-(naphthylethyl)glycine residues.Org Lett. 2013 Oct 4;15(19):5118-21. doi: 10.1021/ol4025502. Epub 2013 Sep 19. Org Lett. 2013. PMID: 24050710
-
Strategies to Control the Cis-Trans Isomerization of Peptoid Amide Bonds.Chem Asian J. 2022 Jun 1;17(11):e202200149. doi: 10.1002/asia.202200149. Epub 2022 Apr 20. Chem Asian J. 2022. PMID: 35362652 Review.
-
Peptide science: A "rule model" for new generations of peptidomimetics.Acta Biomater. 2020 Jan 15;102:35-74. doi: 10.1016/j.actbio.2019.10.045. Epub 2019 Nov 5. Acta Biomater. 2020. PMID: 31698048 Review.
Cited by
-
Navigating the Expansive Landscapes of Soft Materials: A User Guide for High-Throughput Workflows.ACS Polym Au. 2023 Dec 5;3(6):406-427. doi: 10.1021/acspolymersau.3c00025. eCollection 2023 Dec 13. ACS Polym Au. 2023. PMID: 38107416 Free PMC article. Review.
-
Insights into conformational ensembles of compositionally identical disordered peptidomimetics.Polym Chem. 2024 Aug 7;15(29):2970-2980. doi: 10.1039/D4PY00341A. Epub 2024 Jul 4. Polym Chem. 2024. PMID: 39781370 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

