Combining Nanomaterials and Developmental Pathways to Design New Treatments for Cardiac Regeneration: The Pulsing Heart of Advanced Therapies
- PMID: 32391340
- PMCID: PMC7193099
- DOI: 10.3389/fbioe.2020.00323
Combining Nanomaterials and Developmental Pathways to Design New Treatments for Cardiac Regeneration: The Pulsing Heart of Advanced Therapies
Abstract
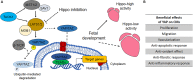
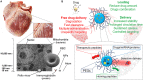
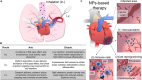
The research for heart therapies is challenged by the limited intrinsic regenerative capacity of the adult heart. Moreover, it has been hampered by the poor results obtained by tissue engineering and regenerative medicine attempts at generating functional beating constructs able to integrate with the host tissue. For this reason, organ transplantation remains the elective treatment for end-stage heart failure, while novel strategies aiming to promote cardiac regeneration or repair lag behind. The recent discovery that adult cardiomyocytes can be ectopically induced to enter the cell cycle and proliferate by a combination of microRNAs and cardioprotective drugs, like anti-oxidant, anti-inflammatory, anti-coagulants and anti-platelets agents, fueled the quest for new strategies suited to foster cardiac repair. While proposing a revolutionary approach for heart regeneration, these studies raised serious issues regarding the efficient controlled delivery of the therapeutic cargo, as well as its timely removal or metabolic inactivation from the site of action. Especially, there is need for innovative treatment because of evidence of severe side effects caused by pleiotropic drugs. Biocompatible nanoparticles possess unique physico-chemical properties that have been extensively exploited for overcoming the limitations of standard medical therapies. Researchers have put great efforts into the optimization of the nanoparticles synthesis and functionalization, to control their interactions with the biological milieu and use as a viable alternative to traditional approaches. Nanoparticles can be used for diagnosis and deliver therapies in a personalized and targeted fashion. Regarding the treatment of cardiovascular diseases, nanoparticles-based strategies have provided very promising outcomes, in preclinical studies, during the last years. Efficient encapsulation of a large variety of cargos, specific release at the desired site and improvement of cardiac function are some of the main achievements reached so far by nanoparticle-based treatments in animal models. This work offers an overview on the recent nanomedical applications for cardiac regeneration and highlights how the versatility of nanomaterials can be combined with the newest molecular biology discoveries to advance cardiac regeneration therapies.
Keywords: Hippo pathway; YAP; cardiac regeneration; cardiomyopathy; nanoparticles; targeted delivery.
Copyright © 2020 Cassani, Fernandes, Vrbsky, Ergir, Cavalieri and Forte.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

