Heterostructured CeO2-M (M = Co, Cu, Mn, Fe, Ni) Oxide Nanocatalysts for the Visible-Light Photooxidation of Pinene to Aroma Oxygenates
- PMID: 32391465
- PMCID: PMC7203698
- DOI: 10.1021/acsomega.9b04396
Heterostructured CeO2-M (M = Co, Cu, Mn, Fe, Ni) Oxide Nanocatalysts for the Visible-Light Photooxidation of Pinene to Aroma Oxygenates
Abstract
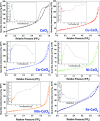
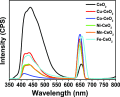
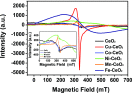
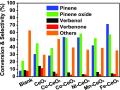
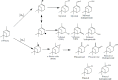
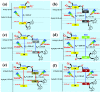
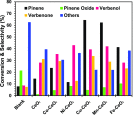
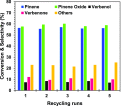
Herein, we report the enhanced photocatalytic activity of heterostructured CeO2 nanocatalysts interfaced with Cu, Co, Ni, Mn, and Fe metal oxides. The CeO2 catalysts exhibited an enhanced red shift in the visible-light response compared to CeO2. This improved absorption range effectively suppressed electron (e-)/hole (+h) recombination by forming localized energy bands associated with defect oxygen vacancies (V o) induced by the Mn+ ions incorporated in CeO2. Under visible-light irradiation, CeO2 catalysts are active for α-pinene oxidation to the aroma oxygenates, pinene oxide, verbenol, and verbenone. Both Fe2O3-CeO2 and NiO-CeO2 gave the highest pinene conversions of 71.3 and 53.1%, respectively, with corresponding pinene oxide selectivities of 57.3 and 58.2%. The enhanced photocatalytic performance of the heterostructured CeO2 catalysts compared to CeO2 is attributed to their enhanced visible-light absorption range and efficient suppression of e-/+h recombination. The Fe2O3-CeO2 catalyst was highly recyclable and did not show any significant loss of its photoactivity.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Cao Y.; Li Y.; Yu H.; Peng F.; Wang H. Aerobic oxidation of α-pinene catalyzed by carbon nanotubes. Catal. Sci. Technol. 2015, 5, 3935–3944. 10.1039/C5CY00136F. - DOI
-
- Ajaikumar S.; Ahlkvist J.; Larsson W.; Shchukarev A.; Leino A. R.; Kordas K.; Mikkola J.-P. Oxidation of α-pinene over gold containing bimetallic nanoparticles supported on reducible TiO2 by deposition-precipitation method. Appl. Catal., A 2011, 392, 11–18. 10.1016/j.apcata.2010.10.015. - DOI
-
- Chen L.; Tang J.; Song L.-N.; Chen P.; He J.; Au C.-T.; Yin S.-F. Heterogeneous photocatalysis for selective oxidation of alcohols and hydrocarbons. Appl. Catal., B 2019, 242, 379–388. 10.1016/j.apcatb.2018.10.025. - DOI
LinkOut - more resources
Full Text Sources

