CO Sense and Release Flavonols: Progress toward the Development of an Analyte Replacement PhotoCORM for Use in Living Cells
- PMID: 32391490
- PMCID: PMC7203955
- DOI: 10.1021/acsomega.0c00409
CO Sense and Release Flavonols: Progress toward the Development of an Analyte Replacement PhotoCORM for Use in Living Cells
Abstract
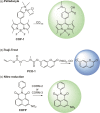
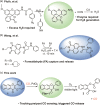
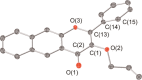
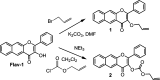
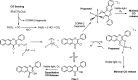
Carbon monoxide (CO) is a signaling molecule in humans. Prior research suggests that therapeutic levels of CO can have beneficial effects in treating a variety of physiological and pathological conditions. To facilitate understanding of the role of CO in biology, molecules that enable fluorescence detection of CO in living systems have emerged as an important class of chemical tools. A key unmet challenge in this field is the development of fluorescent analyte replacement probes that replenish the CO that is consumed during detection. Herein, we report the first examples of CO sense and release molecules that involve combining a common CO-sensing motif with a light-triggered CO-releasing flavonol scaffold. A notable advantage of the flavonol-based CO sense and release motif is that it is trackable via fluorescence in both its pre- and postsensing (pre-CO release) forms. In vitro studies revealed that the PdCl2 and Ru(II)-containing CORM-2 used in the CO sensing step can result in metal coordination to the flavonol, which minimizes the subsequent CO release reactivity. However, CO detection followed by CO release is demonstrated in living cells, indicating that a cellular environment mitigates the flavonol/metal interactions.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
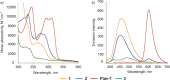
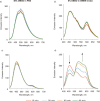
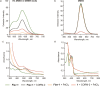
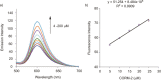


Similar articles
-
Development of Triggerable, Trackable, and Targetable Carbon Monoxide Releasing Molecules.Acc Chem Res. 2020 Oct 20;53(10):2273-2285. doi: 10.1021/acs.accounts.0c00402. Epub 2020 Sep 15. Acc Chem Res. 2020. PMID: 32929957 Free PMC article.
-
Sense and Release: A Thiol-Responsive Flavonol-Based Photonically Driven Carbon Monoxide-Releasing Molecule That Operates via a Multiple-Input AND Logic Gate.J Am Chem Soc. 2017 Jul 19;139(28):9435-9438. doi: 10.1021/jacs.7b04077. Epub 2017 Jul 5. J Am Chem Soc. 2017. PMID: 28677975
-
B-Ring-extended flavonol-based photoCORM: activated by cysteine-ratiometric fluorescence sensing and accurate control of linear CO release.J Mater Chem B. 2021 Oct 13;9(39):8263-8271. doi: 10.1039/d1tb01093j. J Mater Chem B. 2021. PMID: 34499076
-
Carbon Monoxide and Its Controlled Release: Therapeutic Application, Detection, and Development of Carbon Monoxide Releasing Molecules (CORMs).J Med Chem. 2018 Apr 12;61(7):2611-2635. doi: 10.1021/acs.jmedchem.6b01153. Epub 2017 Sep 20. J Med Chem. 2018. PMID: 28876065 Review.
-
Fluorescent nanoprobes for the sensing of gasotransmitters hydrogen sulfide (H2S), nitric oxide (NO) and carbon monoxide (CO).Methods. 2019 Sep 15;168:62-75. doi: 10.1016/j.ymeth.2019.06.003. Epub 2019 Jun 6. Methods. 2019. PMID: 31176771 Review.
Cited by
-
"CO in a pill": Towards oral delivery of carbon monoxide for therapeutic applications.J Control Release. 2021 Oct 10;338:593-609. doi: 10.1016/j.jconrel.2021.08.059. Epub 2021 Sep 2. J Control Release. 2021. PMID: 34481027 Free PMC article. Review.
-
Development of Triggerable, Trackable, and Targetable Carbon Monoxide Releasing Molecules.Acc Chem Res. 2020 Oct 20;53(10):2273-2285. doi: 10.1021/acs.accounts.0c00402. Epub 2020 Sep 15. Acc Chem Res. 2020. PMID: 32929957 Free PMC article.
-
Activity-Based Fluorescent Probes for Hydrogen Sulfide and Related Reactive Sulfur Species.Chem Rev. 2024 Apr 10;124(7):4124-4257. doi: 10.1021/acs.chemrev.3c00683. Epub 2024 Mar 21. Chem Rev. 2024. PMID: 38512066 Free PMC article. Review.
-
Fluorescent small molecule donors.Chem Soc Rev. 2024 Jun 17;53(12):6345-6398. doi: 10.1039/d3cs00124e. Chem Soc Rev. 2024. PMID: 38742651 Free PMC article. Review.
-
A Turn-On Quinazolinone-Based Fluorescence Probe for Selective Detection of Carbon Monoxide.Molecules. 2023 Apr 22;28(9):3654. doi: 10.3390/molecules28093654. Molecules. 2023. PMID: 37175064 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

