Molecular Hybrids Integrated with Benzimidazole and Pyrazole Structural Motifs: Design, Synthesis, Biological Evaluation, and Molecular Docking Studies
- PMID: 32391496
- PMCID: PMC7203960
- DOI: 10.1021/acsomega.0c00630
Molecular Hybrids Integrated with Benzimidazole and Pyrazole Structural Motifs: Design, Synthesis, Biological Evaluation, and Molecular Docking Studies
Abstract
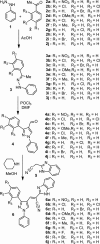
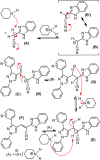
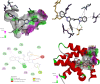
Synthesis of a series of benzimidazole-ornamented pyrazoles, 6a-6j has been obtained from arylhydrazine and aralkyl ketones via a multistep synthetic strategy. Among them, a hybrid-possessing para-nitrophenyl moiety connected to a pyrazole scaffold (6a) exerted the highest anti-inflammatory activity, which is superior to the standard, diclofenac sodium. While executing the 2,2-diphenyl-1-picrylhydrazyl radical-scavenging activity, a hybrid-possessing para-bromophenyl unit integrated at the pyrazole structural motif (6i) exhibited the highest activity among the hybrids examined. Besides, evaluation of anticancer potency of the synthesized hybrids revealed that the one containing a para-fluorophenyl unit tethered at the pyrazole nucleus (6h) showed the highest activity against both the pancreatic cancer cells (SW1990 and AsPCl) investigated. Considerable binding affinity between B-cell lymphoma and the hybrid, 6h has been reflected while performing molecular docking studies (-8.65 kcal/mol). The outcomes of the investigation expose that these hybrids could be used as effective intermediates to construct more potent biological agents.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- https://www.who.int/news-room/fact-sheets, 2018. (accessed 2018-09-12).
-
- Hernández-Covarrubias C.; Vilchis-Reyes M. A.; Yépez-Mulia L.; Sánchez-Díaz R.; Navarrete-Vázquez G.; Hernández-Campos A.; Castillo R.; Hernández-Luis F. Exploring the interplay of physicochemical properties, membrane permeability and giardicidal activity of some benzimidazole derivatives. Eur. J. Med. Chem. 2012, 52, 193–204. 10.1016/j.ejmech.2012.03.014. - DOI - PubMed
-
- Akhtar M. J.; Khan A. A.; Ali Z.; Dewangan R. P.; Rafi M.; Hassan M. Q.; Akhtar M. S.; Siddiqui A. A.; Partap S.; Pasha S.; Yar M. S. Synthesis of stable benzimidazole derivatives bearing pyrazole as anticancer and EGFR receptor inhibitors. Bioorg. Chem. 2018, 78, 158–169. 10.1016/j.bioorg.2018.03.002. - DOI - PubMed
LinkOut - more resources
Full Text Sources

