Exocyst mutants suppress pollen tube growth and cell wall structural defects of hydroxyproline O-arabinosyltransferase mutants
- PMID: 32391581
- PMCID: PMC7496944
- DOI: 10.1111/tpj.14808
Exocyst mutants suppress pollen tube growth and cell wall structural defects of hydroxyproline O-arabinosyltransferase mutants
Abstract
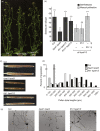
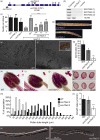
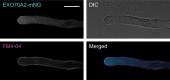
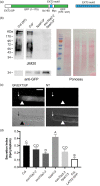
HYDROXYPROLINE O-ARABINOSYLTRANSFERASEs (HPATs) initiate a post-translational protein modification (Hyp-Ara) found abundantly on cell wall structural proteins. In Arabidopsis thaliana, HPAT1 and HPAT3 are redundantly required for full pollen fertility. In addition to the lack of Hyp-Ara in hpat1/3 pollen tubes (PTs), we also found broadly disrupted cell wall polymer distributions, particularly the conversion of the tip cell wall to a more shaft-like state. Mutant PTs were slow growing and prone to rupture and morphological irregularities. In a forward mutagenesis screen for suppressors of the hpat1/3 low seed-set phenotype, we identified a missense mutation in exo70a2, a predicted member of the vesicle-tethering exocyst complex. The suppressed pollen had increased fertility, fewer morphological defects and partially rescued cell wall organization. A transcriptional null allele of exo70a2 also suppressed the hpat1/3 fertility phenotype, as did mutants of core exocyst complex member sec15a, indicating that reduced exocyst function bypassed the PT requirement for Hyp-Ara. In a wild-type background, exo70a2 reduced male transmission efficiency, lowered pollen germination frequency and slowed PT elongation. EXO70A2 also localized to the PT tip plasma membrane, consistent with a role in exocyst-mediated secretion. To monitor the trafficking of Hyp-Ara modified proteins, we generated an HPAT-targeted fluorescent secretion reporter. Reporter secretion was partially dependent on EXO70A2 and was significantly increased in hpat1/3 PTs compared with the wild type, but was reduced in the suppressed exo70a2 hpat1/3 tubes.
Keywords: Arabidopsis thaliana; cell wall; exocyst; glycoprotein; pollen tube; secretion; tip growth.
© 2020 The Authors. The Plant Journal published by Society for Experimental Biology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Adams, E. and Frank, L. (1980) Metabolism of proline and the hydroxyprolines. Annu Rev Biochem. 49, 1005–1061. - PubMed
-
- Alonso, J.M. , Stepanova, A.N. , Leisse, T.J. et al . (2003) Genome‐wide insertional mutagenesis of Arabidopsis thaliana. Science, 301, 653–657. - PubMed
-
- Bate, N. and Twell, D. (1998) Functional architecture of a late pollen promoter: pollen‐specific transcription is developmentally regulated by multiple stage‐specific and co‐dependent activator elements. Plant Mol. Biol. 37, 859–869. - PubMed
-
- Beuder, S. and MacAlister, C.A. (2020) Isolation and cloning of suppressor mutants with improved pollen fertility (2020) In: Pollen and Pollen Tube Biology: Methods and Protocols (Geitmann A., ed). MIMB, Humana Press. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

