Opportunities and challenges for antisense oligonucleotide therapies
- PMID: 32391605
- PMCID: PMC7891411
- DOI: 10.1002/jimd.12251
Opportunities and challenges for antisense oligonucleotide therapies
Abstract
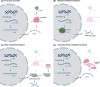
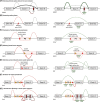
Antisense oligonucleotide (AON) therapies involve short strands of modified nucleotides that target RNA in a sequence-specific manner, inducing targeted protein knockdown or restoration. Currently, 10 AON therapies have been approved in the United States and Europe. Nucleotides are chemically modified to protect AONs from degradation, enhance bioavailability and increase RNA affinity. Whereas single stranded AONs can efficiently be delivered systemically, delivery of double stranded AONs requires capsulation in lipid nanoparticles or binding to a conjugate as the uptake enhancing backbone is hidden in this conformation. With improved chemistry, delivery vehicles and conjugates, doses can be lowered, thereby reducing the risk and occurrence of side effects. AONs can be used to knockdown or restore levels of protein. Knockdown can be achieved by single stranded or double stranded AONs binding the RNA transcript and activating RNaseH-mediated and RISC-mediated degradation respectively. Transcript binding by AONs can also prevent translation, hence reducing protein levels. For protein restoration, single stranded AONs are used to modulate pre-mRNA splicing and either include or skip an exon to restore protein production. Intervening at a genetic level, AONs provide therapeutic options for inherited metabolic diseases as well. This review provides an overview of the different AON approaches, with a focus on AONs developed for inborn errors of metabolism.
Keywords: RNA therapeutics; antisense oligonucleotides; personalized medicine; splicing modulation; targeted gene knockdown; therapies.
© 2020 The Authors. Journal of Inherited Metabolic Disease published by John Wiley & Sons Ltd on behalf of SSIEM.
Conflict of interest statement
A. A. R. discloses being employed by LUMC which has patents on exon skipping technology, some of which has been licensed to BioMarin and subsequently sublicensed to Sarepta. As co‐inventor of some of these patents A. A. R. is entitled to a share of royalties. A. A. R. further discloses being ad hoc consultant for PTC Therapeutics, Sarepta Therapeutics, CRISPR Therapeutics, Summit PLC, Alpha Anomeric, BioMarin Pharmaceuticals Inc., Eisai, Astra Zeneca, Santhera, Audentes, Global Guidepoint and GLG consultancy, Grunenthal, Wave and BioClinica, having been a member of the Duchenne Network Steering Committee (BioMarin) and being a member of the scientific advisory boards of ProQR, Sarepta Therapeutics, Silence Therapeutics and Philae Pharmaceuticals. Remuneration for these activities is paid to LUMC. LUMC also received speaker honoraria from PTC Therapeutics and BioMarin Pharmaceuticals and funding for contract research from Italpharmaco, Zakini and Alpha Anomeric. E. K., A. B. and W. W. P. P. declare no conflict of interest.
Figures
References
-
- Stix G. Shutting down a gene. Antisense drug wins approval. Sci Am. 1998;279(46):50. - PubMed
-
- Hair P, Cameron F, McKeage K. Mipomersen sodium: first global approval. Drugs. 2013;73:487‐493. - PubMed
-
- Aartsma‐Rus A. FDA approval of Nusinersen for spinal muscular atrophy makes 2016 the year of splice modulating oligonucleotides. Nucl Acid Ther. 2017;27:67‐69. - PubMed
-
- Benson MD, Waddington‐Cruz M, Berk JL, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med. 2018;379:22‐31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

