Cost-effectiveness of multi-layered silicone foam dressings for prevention of sacral and heel pressure ulcers in high-risk intensive care unit patients: An economic analysis of a randomised controlled trial
- PMID: 32391627
- PMCID: PMC7948587
- DOI: 10.1111/iwj.13390
Cost-effectiveness of multi-layered silicone foam dressings for prevention of sacral and heel pressure ulcers in high-risk intensive care unit patients: An economic analysis of a randomised controlled trial
Abstract
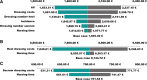
Pressure ulcer incidence is high in intensive care units. This causes a serious financial burden to healthcare systems. We evaluated the cost-effectiveness of multi-layered silicone foam dressings for prevention of sacral and heel pressure ulcers in addition to standard prevention in high-risk intensive care units patients. A randomised controlled trial to assess the efficacy of multi-layered silicone foam dressings to prevent the development of pressure ulcers on heels and sacrum among 422 intensive care unit patients was conducted. Direct costs for preventive dressings in the intervention group and costs for treatment of incident pressure ulcers in both groups were measured using a bottom-up approach. A cost-effectiveness analysis by calculating the incremental cost-effectiveness ratio using different assumptions was performed. Additional dressing and labour costs of €150.81 (€116.45 heels; €34.36 sacrum) per patient occurred in the intervention group. Treatment costs were €569.49 in the control group and €134.88 in the intervention group. The incremental cost-effectiveness ratio was €1945.30 per PU avoided (€8144.72 on heels; €701.54 sacrum) in the intervention group. We conclude that application of preventive dressings is cost-effective for the sacral area, but only marginal on heels for critically ill patients.
Keywords: cost-effectiveness; costs analysis; pressure ulcer; prevention; preventive dressings.
© 2020 The Authors. International Wound Journal published by Medicalhelplines.com Inc and John Wiley & Sons Ltd.
Conflict of interest statement
W. V. P. is a principal consultant for Monument Analytics,and on the scientific advisory board of Mölnlycke Health Care AB. J. K. received consultancy fees from Mölnlycke Health Care AB. All other authors declare no conflicts of interest.
The underlying study was an investigator initiated trial conducted by the Clinical Research Center for Hair and Skin Science, Department of Dermatology and Allergy, Charité – Universitätsmedizin Berlin on behalf of the Clinical Quality and Risk Management of the Charité – Universitätsmedizin Berlin. The study was financially supported by Mölnlycke Health Care AB. Mölnlycke provided the dressings Mepilex Border Sacrum and Mepilex Border Heel for the intervention group. Mölnlycke was not involved at any stage in the data collection and analysis of this project or in the preparation of this manuscript.
Figures
References
-
- European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel and Pan Pacific Pressure Injury Alliance . In: Haesler E, ed. Prevention and Treatment of Pressure Ulcers/Injuries: Clinical Practice Guideline. The International Guideline. EPUAP/NPIAP/PPPIA; 2019.
-
- Lyder CH, Wang Y, Metersky M, et al. Hospital‐acquired pressure ulcers: results from the national medicare patient safety monitoring system study. J Am Geriatr Soc. 2012;60(9):1603‐1608. - PubMed
-
- National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel and Pan Pacific Pressure Injury Alliance . In: Haesler E, ed. Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline. Osborne Park, Australia: Cambridge Media; 2014.
-
- Tomova‐Simitchieva T, Akdeniz M, Blume‐Peytavi U, Lahmann N, Kottner J. The epidemiology of pressure ulcer in Germany: systematic review. Gesundheitswesen. 2019;81(6):505‐512. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

